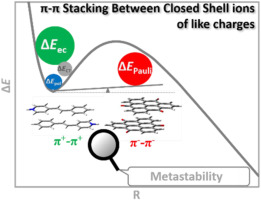
Planar cations or anions can form stacks in crystals or solutions, where the surrounding or environment plays a decisive role as demonstrated in previous studies. However, it remains unclear whether these counterintuitive interactions possess any inherent stability or are thoroughly repulsive if the constraint of environment is removed. In this work, we explored the inherent stability of π-π stacking between closed-shell ions of like charges with prototypes derived from experimental studies. The inherent metastability was identified by the characteristic local minima and the transition states preventing their dissociation and verified by ab initio molecular dynamics (AIMD) simulations. The nature of involved interactions was deciphered with the energy decomposition approach based on the block-localized wavefunction method (BLW-ED). Like the conventional neutral π-π stacking interactions, electron correlation is the most attractive energy component. But it is overturned by the Coulombic repulsion between net charges for all modes of dimerization, resulting in the overall repulsive inter-cation or anion interactions. Contributions from van der Waals interactions were also observed in the reduced density gradient analysis. The origin of the metastability was elucidated by examining the contributions of individual physical factors to the well-depths. The inherent metastability originates from the electron correlation, which dramatically increases due to the enhanced overlap between ions from a transition state to its corresponding minimum.