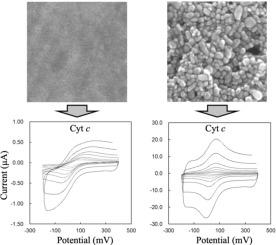
Electrochemical manipulation of enzymes is expected to enable the rapid detection of marker molecules and the efficient production of valuable materials. We have fabricated an Au nanoparticle (AuNP) thin film electrode in our previous study, which enables more enhanced heterogeneous electron transfer reactions than a conventional planner Au electrode. In this study, electrochemical evaluation of cytochrome c was performed using the AuNP thin film electrodes with and without self-assembled monolayer (SAM) modification to assess its practicality for protein electrochemistry. The 4-pyridinethiol- and 7-carboxy-1-heptanethiol-modified AuNP thin film electrodes showed much larger faradaic current values attributed to redox reactions compared to those of the Au electrodes. The results indicate that the nanostructural effects of the AuNP thin film electrode are beneficial. Importantly, even when the non-modified (bare) AuNP thin film electrode was applied, it exhibited the clear electrochemical response of cyt c, while the bare Au electrode showed no such a response. This study provides evidence that the AuNP thin film electrode functions as a potent bioelectrocatalysts due to the nanostructure-specific properties.