Exploring an innovative method for precious metal recovery from spent batteries, our study combines hydrometallurgy and pyrometallurgy, focusing on pivotal carbothermal reduction. This crucial phase enhances the leachability of metals by significantly reducing their valence. To evaluate the effect of novel carbon (C) materials on this reduction process, our study synthesized two types of nanoporous Cs, Al-PCP-800 and ZIF-8-800, found in electrode materials. Their carbothermal reduction efficacy was compared to activated (A)C using thermogravimetric analysis.
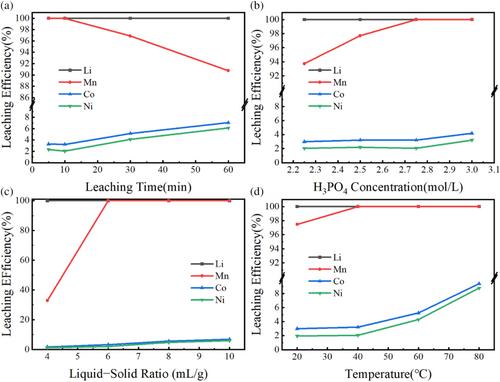
The experimental findings revealed that ZIF-8-800 exhibits superior reducibility, initiating and reaching peak weight loss rate at a lower temperature of 692.01 °C with a peak of 32.27%/°C. Under the optimized conditions (roasting temperature 750 °C, time 3 h, C content 20%, 2.75 mol L−1 H3PO4 concentration, 40 °C leaching temperature, liquid-to-solid ratio of 6 mL g−1, leaching time 10 min) the leaching efficiency of lithium (Li) and manganese (Mn) reached 100%, indicating complete extraction with ZIF-8-800. However, cobalt (Co) and nickel (Ni) leaching with ZIF-8-800 was low (3.22%, 2.06%). Al-PCP-800 showed slightly less leaching efficiency for Li and Mn, but higher efficiency for Co and Ni. Activated C led to incomplete Li and Mn extraction, with higher Co and Ni leaching, diminishing the process's selectivity.
The study confirms that the C material in carbothermal reduction significantly impacts metal leaching efficiency and selectivity. ZIF-8-800 was the most effective for Li and Mn leaching, outperforming Al-PCP-800 and AC in selectivity. This underscores ZIF-8-800's potential to improve metal recovery from spent batteries. © 2024 The Authors. Journal of Chemical Technology and Biotechnology published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry (SCI).