Active cigarette smoking is a major risk factor for chronic obstructive pulmonary disease that remains elevated after cessation. Skeletal muscle dysfunction has been well documented after smoking, but little is known about cardiac adaptations to cigarette smoking. The underlying cellular and molecular cardiac adaptations, independent of confounding lifestyle factors, and time course of reversibility by smoking cessation remain unclear. We hypothesized that smoking negatively affects cardiac metabolism and induces local inflammation in mice, which do not readily reverse upon 2-week smoking cessation.
Mice were exposed to air or cigarette smoke for 14 weeks with or without 1- or 2-week smoke cessation. We measured cardiac mitochondrial respiration by high-resolution respirometry, cardiac mitochondrial density, abundance of mitochondrial supercomplexes by electrophoresis, and capillarization, fibrosis, and macrophage infiltration by immunohistology, and performed cardiac metabolome and lipidome analysis by mass spectrometry.
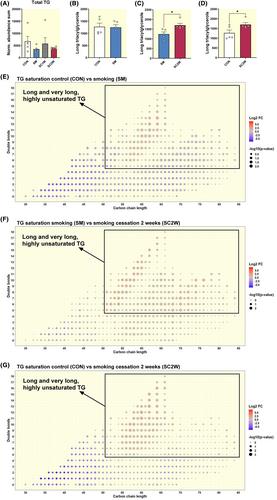
Mitochondrial protein, supercomplex content, and respiration (all p < 0.03) were lower after smoking, which were largely reversed within 2-week smoking cessation. Metabolome and lipidome analyses revealed alterations in mitochondrial metabolism, a shift from fatty acid to glucose metabolism, which did not revert to control upon smoking cessation. Capillary density was not different after smoking but increased after smoking cessation (p = 0.02). Macrophage infiltration and fibrosis (p < 0.04) were higher after smoking but did not revert to control upon smoking cessation.
While cigarette-impaired smoking-induced cardiac mitochondrial function was reversed by smoking cessation, the remaining fibrosis and macrophage infiltration may contribute to the increased risk of cardiovascular events after smoking cessation.