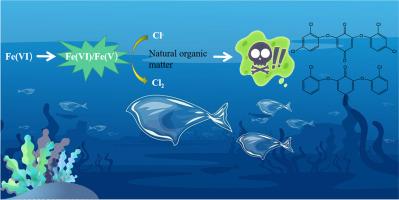
Currently, ferrate(VI) oxidation technology (FOT) has been regarded as one of the most promising options for the degradation of emerging organic pollutants. However, the role and transformation of chloride ions (Cl−) in FOT have not been well explored. The current study aims to investigate the formation of chlorinated phenolic byproducts upon ferrate(VI) oxidation processes. The obtained results indicate that chlorides suffering ferrate(VI) attack will be transformed to active chlorine species (ACS), which will subsequently lead to the formation of highly toxic aromatic chlorinated byproducts. The identified byproducts include common chlorinated phenolic derivatives, as well as complex chlorinated oligomer byproducts with ether structures (mainly dimers and trimers). While the formation of common chlorophenols can be ascribed to the electrophilic substitution reactions mediated by ACS, the oligomer byproducts are generated via coupling reactions between chlorinated phenoxy radicals. ECOSAR software predicts that the generated chlorinated oligomer byproducts exhibit high ecotoxicological effects. As a whole, the above findings shed light on the potential risk of FOT in real practice.
| 公司名称 | 产品信息 | 采购帮参考价格 | |
|---|---|---|---|
| 上海源叶 | potassium ferrate |
99.5%
|
¥15.00~¥10479.00 |


