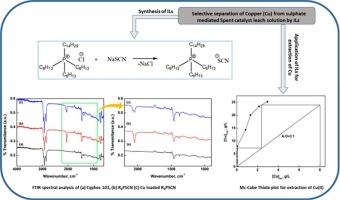
Separation and recovery of copper (Cu) from sulphate mediated CuCr spent catalyst leach solution through solvent extraction approach has been systematically investigated. A number of Ionic Liquid (IL) and other conventional extractants were used while investigating the selectivity and efficient extraction tendency of either IL or extractants towards Cu(II). In context of copper extraction efficiency, the adopted organic reagents followed the descending order: R4PSCN > R4PD > R4PCy > Cyphos 101 > Aliquot 336 > D2EHPA > Cyanex 272. The extraction behavior of Cu(II) was established based on slope analysis method. From the results it was noticed that extraction occurs through a cation exchange mechanism with association of a mole of Cu(II) per mole of R4PSCN. The plot of log D vs. log [R4PSCN] yield a linear relationship with a slope close to 4 which is used to propose extraction reaction mechanism/ stoichiometry. The nature of complex between Cu(II) and IL was further examined using FTIR analysis of the loaded organic phase with the diluent. Mc-Cabe Thiele diagram was constructed to predict quantitative extraction of Cu(II) which revealed the need for two stages at aqueous to organic (A:O) phase volume ratio of =3:1. The stripping isotherm constructed at optimum NH4OH concentration (0.4 M) suggests the need for two stages at O:A phase volume ratio of = 2:1 for complete stripping of copper with regeneration of R4SCN for further use. Both isotherm conditions were validated by 6 cycles of counter current simulation (CCS) study for obtaining the desired amount of Cu(II) loaded R4SCN (during extraction) and/or stripped copper solution (during stripping). Overall copper enrichment was ∼6 fold leading to produce a copper(II) solution of 48 g/L from 6 g/L Cu(II). The stripped solution was subjected to crystallization study to produce copper sulphate crystals of high purity and was confirmed by XRD analysis of crystal phases.