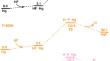
To understand the detailed reaction kinetics and mechanism of the reaction between Hg and HF, theoretical investigations of their reactions at different temperatures were carried out. The results suggest that the reactions goes through two steps. In the first step, Hg interacts with HF to form a complex HF⋯Hg, and then the F atom of HF approaches to Hg to form the transition state H∙∙∙F∙∙∙Hg, the bonding between F and Hg atoms results in the formation of HgF. Subsequently, the second HF molecule takes part in and it interacts with HgF to form the intermediate HF∙∙∙HgF, and then the transition state H∙∙∙F∙∙∙HgF forms due to the approaching of F atom of HF to Hg atom of HgF, finally the product HgF2 is produced after the F and Hg atoms are bonded. The temperature significantly influences the reaction process. The weak interaction in the formation of the complex HF∙∙∙Hg as well as the intermediate HF∙∙∙HgF was illustrated by quantum theory of atoms in molecules (QTAIM). The kinetic parameters including the pre-exponential factor A, activation energy Ea and reaction rate k at different temperatures were calculated, and the expressions of reaction rates k for the reactions between HF and Hg to form HgF as well as HgF2 were derived. The results would provide valuable insights into the chemical reaction of Hg and HF, the mechanism and the kinetics.