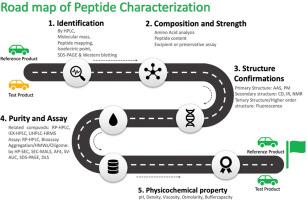
The Peptide therapeutics market was evaluated to be around USD 45.67 BN in 2023 and is projected to witness massive growth at a CAGR of around 5.63 % from 2024 to 2032 (USD 80.4 BN). Generic peptides are expected to reach USD 27.1 billion by 2032 after the patent monopoly of the pioneer peptides expires, and generic peptides become accessible. The generic manufacturers are venturing into peptide-based therapeutics for the aforementioned reasons. There is an abundance of material accessible regarding the characterization of peptides, which can be quite confusing for researchers. The FDA believes that an ANDA applicant may now demonstrate that the active component in a proposed generic synthetic peptide drug product is the “same” as the active ingredient in a peptide of rDNA origin that has previously been approved. To ensure the efficacy, safety, and quality of peptide therapies during development, regulatory bodies demand comprehensive characterization utilizing several orthogonal methodologies. This article elaborates the peptide characterization by segmenting into different segments as per the critical quality attribute from identification of the peptide to the physicochemical property of the peptide therapeutics which will be required to demonstrate the sameness with reference product based on the size of the peptide chain and molecular weight of the peptides. Article insights briefly on each individual technique and the orthogonal techniques for each test were explained. The impurities requirements in the generic peptides as per the regulatory requirement were also discussed.