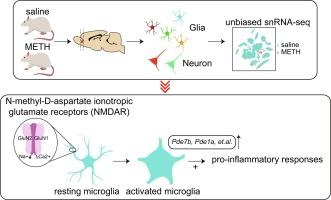
The behavioral sensitization, characterized by escalated behavioral responses triggered by recurrent exposure to psychostimulants, involves neurobiological mechanisms that are brain-region and cell-type specific. Enduring neuroadaptive changes have been observed in response to methamphetamine (METH) within the orbitofrontal cortex (OFC), the cell-type specific transcriptional alterations in response to METH sensitization remain understudied. In this study, we utilized Single-nucleus RNA-sequencing (snRNA-seq) to profile the gene expression changes in the OFC of a rat METH sensitization model. The analyses of differentially expressed genes (DEGs) unveiled cell-type specific transcriptional reactions associated with METH sensitization, with the most significant alterations documented in microglial cells. Bioinformatic investigations revealed that distinct functional and signaling pathways enriched in microglia-specific DEGs majorly involved in macroautophagy processes and the activation of N-methyl-D-aspartate ionotropic glutamate receptors (NMDAR). To validate the translational relevance of our findings, we analyzed our snRNA-seq data in conjunction with a transcriptomic study of individuals with opioid use disorder (OUD) and a large-scale Genome-Wide Association Studies (GWAS) from multiple externalizing phenotypes related to drug addiction. The validation analysis confirmed the consistent expression changes of key microglial DEGs in human METH addiction. Moreover, the integration with GWAS data revealed associations between addiction risk genes and the DEGs observed in specific cell types, particularly microglia and excitatory neurons. Our study highlights the importance of cell-type specific transcriptional alterations in the OFC in the context of METH sensitization and their potential translational relevance to human drug addiction.