The impact of continuous flow resulting from contemporary left ventricular assist devices (LVAD) on renal vascular physiology is unknown. Renal resistive index (RRI) reflects arterial compliance, as well as renal vascular resistance, contributed by afferent and efferent arteriolar tone, the renal interstitium as well as renal venous pressures.
Prospective, single center study with renal Doppler evaluation at baseline (pre-implant) and at 3-months support. Outcomes assessed include need for post-operative renal replacement therapy (RRT), worsening renal function (WRF) defined as persistent increase from pre-implant KDIGO chronic kidney disease stage, right ventricular (RV) failure, and survival to transplantation.
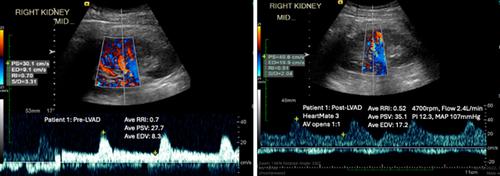
Pre-implant RRI did not predict cardiorenal outcomes including right heart failure, need for renal replacement therapy or worsening renal function. Post-implant RRI was significantly lower than pre-implant RRI, with a distinct Doppler waveform characteristic of continuous flow. Post-implant renal end-diastolic velocity, but not RRI, correlated strongly with LVAD flow (Spearman rho −0.99, p < 0.001), with trend toward correlation with mean arterial pressure (Spearman's rho 0.63, p = 0.129). There was a negative correlation between post-implant RRI and mean pulmonary artery pressure (Spearman's rho −0.81, p = 0.049), likely driven by elevated pulmonary capillary wedge pressure (Spearman's rho −0.83, p = 0.058).
The hemodynamic contributors to RRI in LVAD supported patients are complex. Higher mean pulmonary artery and pulmonary capillary wedge pressures seen in lower RRI may reflect a smaller difference in systolic and diastolic flow. Future simultaneous Doppler assessment of the LVAD outflow graft and RRI may help understand the hemodynamic interactions contributing to this index.