Pancreatitis is a severe inflammatory pathology that occurs from pancreatic duct and exocrine acinar injury, leading to improper secretion of digestive enzymes, auto-digestion of the pancreas, and subsequent inflammation. Clinical reports show that 60%–90% of pancreatitis patients have a history of chronic alcohol use. More recent studies reveal that exocrine pancreas disorders like acute pancreatitis can precede diabetes type II onset, though mechanisms are not yet fully known. This study identified molecules and key signaling pathways underlying alcohol-induced acute pancreatitis and their effects on diabetes type II onset.
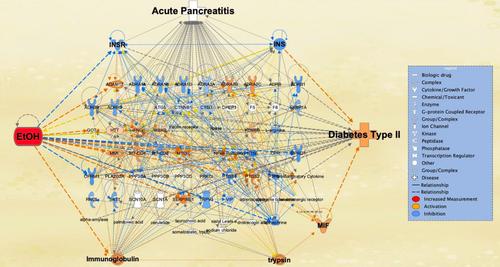
Data on human peripheral blood samples with or without acute pancreatitis were retrieved from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (accession number GSE194331). Acute pancreatitis-mediated differentially expressed genes (DEGs) were generated from GSE194331 using CLC Genomics Workbench 12. Molecules associated with ethanol (EtOH), acute pancreatitis, and diabetes type II were collected from QIAGEN Knowledge Base (QKB). The relationship between the molecules and signaling pathways associated with EtOH, acute pancreatitis, or diabetes type II was examined using various Ingenuity Pathway Analysis (IPA) tools.
Our investigation showed that acute pancreatitis-mediated DEGs were closely associated with EtOH by revealing that EtOH-induced acute pancreatitis appears to lead to the onset of diabetes type II. We found that diabetes type II onset was mediated by pro-inflammatory and metabolic mechanisms underlying EtOH-induced acute pancreatitis, involving increased expression of cytokines including macrophage migration inhibitory factor (MIF), and decreased expression of hormones such as insulin.
Exposure to alcohol may promote diabetes type II by affecting the activity of key inflammatory and metabolic mediators involved in acute pancreatitis. These findings call for further investigation into the role of pro-inflammatory and metabolic mediators like resistin, IL-6, and insulin in EtOH-induced diabetes type II associated with acute pancreatitis pathologies.