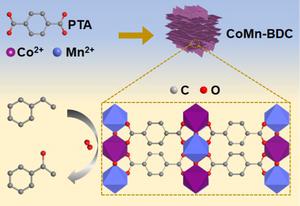
The selective oxidation of hydrocarbons can be used to produce oxygen-containing functional compounds such as alcohols, aldehydes or ketones and its efficient and green conversion lies in the development of efficient catalysts that activate C-H bonds and O2 simultaneously. In this work, the bimetallic organic framework (CoMnBDC) material with morphology of stacked nanosheets was synthesized using terephthalic acid as ligands to coordinate with Co2+ and Mn2+ cations under solvothermal conditions. As revealed by spectroscopic characterizations, the electron transfer from Mn to Co in the CoMnBDC resulted in the reduction of the Co average oxidation state and increase of the Mn average oxidation state. The CoMnBDC nanosheets were used as catalyst in catalytic oxidation of ethylbenzene, in which the redox effect promotes the effective electron transfer, the activation of O2 and benzyl C-H bond. The 96.2% conversion of ethylbenzene and 98.0% selectivity towards acetophenone could be obtained with oxygen as sole oxidant and solvent-free condition. The excellent catalytic performance is related to the structure of CoMnBDC and is also the best when compared with reported results. Various types of aromatic hydrocarbons containing benzyl C-H bonds can be effectively oxidized by CoMnBDC to produce corresponding ketone products. The density functional theory (DFT) calculation revealed that the redox effect leads to the relative enrichment of electrons on Co in CoMnBDC, which is conducive to the activation of O2; Mn with higher oxidation state is beneficial for the adsorption of ethylbenzene and activation of C-H bonds. The CoMnBDC has a lower energy barrier for transition state, making it easier for the ethylbenzene oxidation to produce acetophenone.