The evolutionary history of metastatic pancreatic neuroendocrine tumours reveals a therapy driven route to high-grade transformation
Samuel Backman, Johan Botling, Helena Nord, Suman Ghosal, Peter Stålberg, C. Christofer Juhlin, Jonas Almlöf, Anders Sundin, Liang Zhang, Lotte Moens, Barbro Eriksson, Staffan Welin, Per Hellman, Britt Skogseid, Karel Pacak, Kazhan Mollazadegan, Tobias Åkerström, Joakim Crona
下载PDF
{"title":"The evolutionary history of metastatic pancreatic neuroendocrine tumours reveals a therapy driven route to high-grade transformation","authors":"Samuel Backman, Johan Botling, Helena Nord, Suman Ghosal, Peter Stålberg, C. Christofer Juhlin, Jonas Almlöf, Anders Sundin, Liang Zhang, Lotte Moens, Barbro Eriksson, Staffan Welin, Per Hellman, Britt Skogseid, Karel Pacak, Kazhan Mollazadegan, Tobias Åkerström, Joakim Crona","doi":"10.1002/path.6348","DOIUrl":null,"url":null,"abstract":"<p>Tumour evolution with acquisition of more aggressive disease characteristics is a hallmark of disseminated cancer. Metastatic pancreatic neuroendocrine tumours (PanNETs) in particular may progress from a low/intermediate to a high-grade disease. The aim of this work was to understand the molecular mechanisms underlying metastatic progression as well as PanNET transformation from a low/intermediate to a high-grade disease. We performed multi-omics analysis (genome/exome sequencing, total RNA-sequencing and methylation array) of 32 longitudinal samples from six patients with metastatic low/intermediate grade PanNET. The clonal composition of tumour lesions and underlying phylogeny of each patient were determined with bioinformatics analyses. Findings were validated in post-alkylating chemotherapy samples from 24 patients with PanNET using targeted next generation sequencing. We validate the current PanNET evolutionary model with <i>MEN1</i> inactivation that occurs very early in tumourigenesis. This was followed by pronounced genetic diversity on both spatial and temporal levels, with parallel and convergent tumour evolution involving the ATRX/DAXX and mechanistic target of the rapamycin (mTOR) pathways. Following alkylating chemotherapy treatment, some PanNETs developed mismatch repair deficiency and acquired a hypermutational phenotype. This was validated among 16 patients with PanNET who had high-grade progression after alkylating chemotherapy, of whom eight had a tumour mutational burden >50 (50%). In comparison, among the eight patients who did not show high-grade progression, 0 had a tumour mutational burden >50 (0%; odds ratio ‘infinite’, 95% confidence interval 1.8 to ‘infinite’, <i>p</i> = 0.02). Our findings contribute to broaden the understanding of metastatic/high-grade PanNETs and suggests that therapy driven disease evolution is an important hallmark of this disease. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 4","pages":"357-370"},"PeriodicalIF":5.2000,"publicationDate":"2024-10-03","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6348","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://pathsocjournals.onlinelibrary.wiley.com/doi/10.1002/path.6348","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
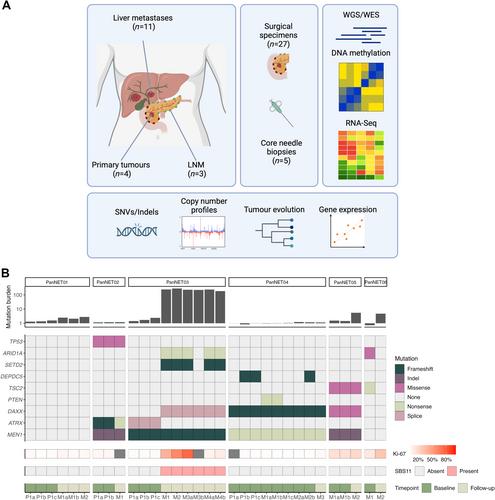
Tumour evolution with acquisition of more aggressive disease characteristics is a hallmark of disseminated cancer. Metastatic pancreatic neuroendocrine tumours (PanNETs) in particular may progress from a low/intermediate to a high-grade disease. The aim of this work was to understand the molecular mechanisms underlying metastatic progression as well as PanNET transformation from a low/intermediate to a high-grade disease. We performed multi-omics analysis (genome/exome sequencing, total RNA-sequencing and methylation array) of 32 longitudinal samples from six patients with metastatic low/intermediate grade PanNET. The clonal composition of tumour lesions and underlying phylogeny of each patient were determined with bioinformatics analyses. Findings were validated in post-alkylating chemotherapy samples from 24 patients with PanNET using targeted next generation sequencing. We validate the current PanNET evolutionary model with MEN1 inactivation that occurs very early in tumourigenesis. This was followed by pronounced genetic diversity on both spatial and temporal levels, with parallel and convergent tumour evolution involving the ATRX/DAXX and mechanistic target of the rapamycin (mTOR) pathways. Following alkylating chemotherapy treatment, some PanNETs developed mismatch repair deficiency and acquired a hypermutational phenotype. This was validated among 16 patients with PanNET who had high-grade progression after alkylating chemotherapy, of whom eight had a tumour mutational burden >50 (50%). In comparison, among the eight patients who did not show high-grade progression, 0 had a tumour mutational burden >50 (0%; odds ratio ‘infinite’, 95% confidence interval 1.8 to ‘infinite’, p = 0.02). Our findings contribute to broaden the understanding of metastatic/high-grade PanNETs and suggests that therapy driven disease evolution is an important hallmark of this disease. © 2024 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
转移性胰腺神经内分泌肿瘤的演变史揭示了一条治疗驱动的高级别转化之路。
肿瘤演变并获得更具侵袭性的疾病特征是扩散性癌症的特征。转移性胰腺神经内分泌肿瘤(PanNET)尤其可能从低/中度疾病发展为高度疾病。这项工作的目的是了解转移性进展以及PanNET从低/中度疾病向高级别疾病转化的分子机制。我们对6名转移性低/中度PanNET患者的32份纵向样本进行了多组学分析(基因组/外显子组测序、总RNA测序和甲基化阵列)。通过生物信息学分析确定了每位患者肿瘤病变的克隆组成和潜在的系统发育。利用新一代靶向测序技术对 24 名 PanNET 患者的烷基化化疗后样本进行了验证。我们验证了当前的 PanNET 演化模型,即在肿瘤发生的早期出现 MEN1 失活。随后,在空间和时间层面上都出现了明显的遗传多样性,肿瘤的平行和趋同进化涉及 ATRX/DAXX 和雷帕霉素(mTOR)机制靶点通路。经过烷化疗治疗后,一些 PanNET 出现了错配修复缺陷,并获得了高突变表型。16 名接受烷化化疗后病情出现高级别进展的 PanNET 患者证实了这一点,其中 8 人的肿瘤突变负荷大于 50(50%)。相比之下,在未出现高级别进展的8名患者中,0人的肿瘤突变负荷大于50(0%;几率比为 "无限",95%置信区间为1.8至 "无限",P = 0.02)。我们的研究结果有助于拓宽人们对转移性/高级别 PanNET 的认识,并表明治疗驱动的疾病演变是这种疾病的一个重要特征。© 2024 作者姓名病理学杂志》由约翰威利父子有限公司代表大不列颠及爱尔兰病理学会出版。本文由美国政府雇员撰写,其作品在美国属于公共领域。
本文章由计算机程序翻译,如有差异,请以英文原文为准。