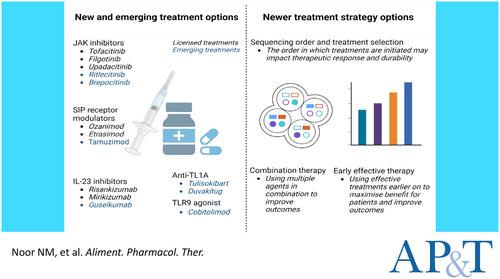
Several new treatments including small molecules and biologics have been approved for the treatment of inflammatory bowel diseases in recent years. Clinicians and patients now have a wide variety of therapeutic options to choose from and these novel therapies provide several advantages including oral administration, lower immunogenicity, better selectivity and arguably better safety profiles. An increase in treatment options has increased the complexity of decision-making. Both patients and clinicians have had to become rapidly familiar with efficacy of new medications balanced against a range of pre-initiation requirements, dosing schedules and adverse event profiles.
To provide a simple guide to practising clinicians on recently approved and emerging therapies and address key challenges around treatment strategies such as optimal sequencing and timing of treatment.
We comprehensively searched the published literature and major conference abstracts to identify phase III placebo-controlled and active comparator trials for Crohn's disease and ulcerative colitis.
Data for recently approved therapies including selective Janus kinase inhibitors, sphingosine-1 receptor modulators and p19 interleukin (IL)-23 inhibitors have demonstrated improved patient outcomes in both Crohn's disease and ulcerative colitis. Further comparative head-to-head studies have improved our understanding of when and how to optimally use newer therapies, specifically for IL-23 inhibitors. Data for emerging treatment options and novel treatment strategies such as early effective treatment, combinations of treatments and implications for sequencing are continuing to transform IBD care continually.
Recently approved novel therapies have expanded the range of medical options available to treat IBD. However, further data from long-term extension studies, real-world studies and head-to-head trials are warranted to better inform the long-term safety and optimal sequencing of treatments for patients living with IBD.