Glucagon-like peptide-1 receptor agonists (GLP-1 RAs), a class of injectable antidiabetic drugs, have shown significant efficacies in improving glycemic and weight control in patients with type 2 diabetes (T2D). However, the long-term safety of GLP-1 RAs remains insufficiently studied. This study aimed to provide real-world evidence on potential adverse outcomes associated with GLP-1 RAs use in T2D patients without major chronic diseases including impaired cardiac or renal function.
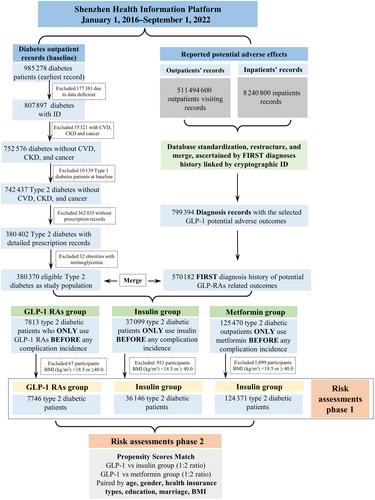
We conducted a retrospective cohort study involving 7746 T2D patients on GLP-1 RAs in Shenzhen, China. They were compared with 124 371 metformin-only users and 36 146 insulin-only users, forming two therapy control groups. GLP-1 RAs users were also further 1:2 paired with the control groups. Competing risk survival analyses were conducted to assess the incidence risks, presenting subdistributional hazard ratios (sHRs) with 95% confidence intervals (CIs) for various adverse outcomes associated with GLP-1 RAs use.
Compared with metformin-only users, GLP-1 RAs use was associated with increased risks of various adverse outcomes (sHRs with 95% CIs), including pancreatitis (2.01, 1.24–3.24), acute nephritis (3.20, 2.17–4.70), kidney failure (3.73, 2.74–5.08), thyroid cancer (2.25, 1.23–4.10), and thyroid dysfunction (1.27, 1.00–1.63), respectively; Similar results were also found when compared with insulin-only users. Importantly, long-term (≥12 months) GLP-1 RAs use may further elevate the incidence risks of pancreatitis, acute nephritis, thyroid cancer, and thyroid dysfunction.
Compared with traditional T2D treatments, GLP-1 RAs use may be associated with increased risks of various adverse outcomes in a Chinese population. Cautions were strongly warranted in the use of GLP-1 RAs. Further validation is crucial across diverse populations.