{"title":"Using Spectral Flow Cytometry to Characterize Anti-Tumor Immunity in Orthotopic and Subcutaneous Mouse Models of Cancer","authors":"Giampiero Valenzano, Shannon N. Russell, Simei Go, Eric O'Neill, Keaton I. Jones","doi":"10.1002/cpz1.70032","DOIUrl":null,"url":null,"abstract":"<p>Mouse models remain at the forefront of immuno-oncology research, providing invaluable insights into the complex interactions between the immune system and developing tumors. While several flow cytometry panels have been developed to study cancer immunity in mice, most are limited in their capacity to address the complexity of anti-cancer immune responses. For example, many of the panels developed to date focus on a restricted number of leukocyte populations (T cells or antigen-presenting cells), failing to include the multitude of other subsets that participate in anti-cancer immunity. In addition, these panels were developed using blood or splenic leukocytes. While the immune composition of the blood or spleen can provide information on systemic immune responses to cancer, it is in the tumor microenvironment (TME) that local immunity takes place. Therefore, we optimized this spectral flow cytometry panel to identify the chief cell types that take part in cancer immunity using immune cells from cancer tissue. We used pancreatic tumors implanted both orthotopically and subcutaneously to demonstrate the panel's flexibility and suitability in diverse mouse models. The panel was also validated in peripheral immune districts (the blood, spleen, and liver of tumor-bearing mice) to allow comparisons between local and systemic anti-tumor immunity. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Tumor induction—Orthotopic</p><p><b>Alternate Protocol</b>: Tumor induction—Subcutaneous</p><p><b>Basic Protocol 2</b>: Preparation of single-cell suspensions from the tumor, spleen, liver, and blood of tumor-bearing mice</p><p><b>Basic Protocol 3</b>: Staining single-cell suspensions from the tumor, spleen, liver, and blood of tumor-bearing mice</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 10","pages":""},"PeriodicalIF":2.2000,"publicationDate":"2024-10-21","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.70032","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpz1.70032","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Abstract
Mouse models remain at the forefront of immuno-oncology research, providing invaluable insights into the complex interactions between the immune system and developing tumors. While several flow cytometry panels have been developed to study cancer immunity in mice, most are limited in their capacity to address the complexity of anti-cancer immune responses. For example, many of the panels developed to date focus on a restricted number of leukocyte populations (T cells or antigen-presenting cells), failing to include the multitude of other subsets that participate in anti-cancer immunity. In addition, these panels were developed using blood or splenic leukocytes. While the immune composition of the blood or spleen can provide information on systemic immune responses to cancer, it is in the tumor microenvironment (TME) that local immunity takes place. Therefore, we optimized this spectral flow cytometry panel to identify the chief cell types that take part in cancer immunity using immune cells from cancer tissue. We used pancreatic tumors implanted both orthotopically and subcutaneously to demonstrate the panel's flexibility and suitability in diverse mouse models. The panel was also validated in peripheral immune districts (the blood, spleen, and liver of tumor-bearing mice) to allow comparisons between local and systemic anti-tumor immunity. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1: Tumor induction—Orthotopic
Alternate Protocol: Tumor induction—Subcutaneous
Basic Protocol 2: Preparation of single-cell suspensions from the tumor, spleen, liver, and blood of tumor-bearing mice
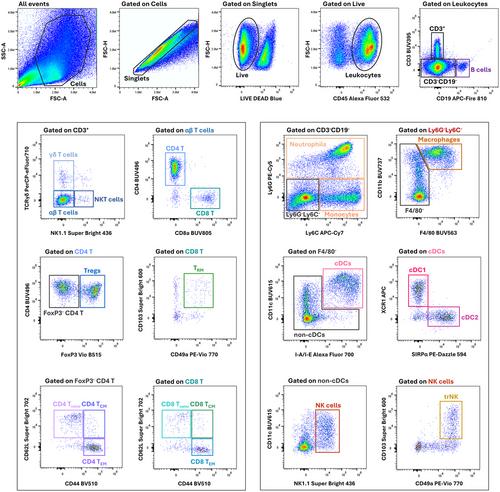
Basic Protocol 3: Staining single-cell suspensions from the tumor, spleen, liver, and blood of tumor-bearing mice

利用光谱流式细胞术鉴定正位和皮下小鼠癌症模型的抗肿瘤免疫特性
小鼠模型仍处于免疫肿瘤学研究的前沿,为了解免疫系统与发展中肿瘤之间复杂的相互作用提供了宝贵的见解。虽然目前已开发出几种流式细胞计数板用于研究小鼠的癌症免疫,但大多数流式细胞计数板在处理复杂的抗癌免疫反应方面能力有限。例如,迄今为止开发的许多面板都只关注数量有限的白细胞群(T 细胞或抗原递呈细胞),而没有包括参与抗癌免疫的众多其他亚群。此外,这些检测板是利用血液或脾脏白细胞开发的。虽然血液或脾脏中的免疫成分可以提供癌症全身免疫反应的信息,但局部免疫是在肿瘤微环境(TME)中发生的。因此,我们优化了这种光谱流式细胞仪面板,利用癌症组织中的免疫细胞来识别参与癌症免疫的主要细胞类型。我们使用了胰腺肿瘤的正位和皮下植入,以证明该面板在不同小鼠模型中的灵活性和适用性。我们还在外周免疫区(携带肿瘤的小鼠的血液、脾脏和肝脏)验证了该小组,以便对局部和全身抗肿瘤免疫进行比较。© 2024 作者。当前协议》由 Wiley Periodicals LLC 出版。基本方案 1:肿瘤诱导-异位替代方案:基本方案 2:制备肿瘤小鼠的肿瘤、脾脏、肝脏和血液中的单细胞悬液 基本方案 3:对肿瘤小鼠的肿瘤、脾脏、肝脏和血液中的单细胞悬液进行染色。
本文章由计算机程序翻译,如有差异,请以英文原文为准。



