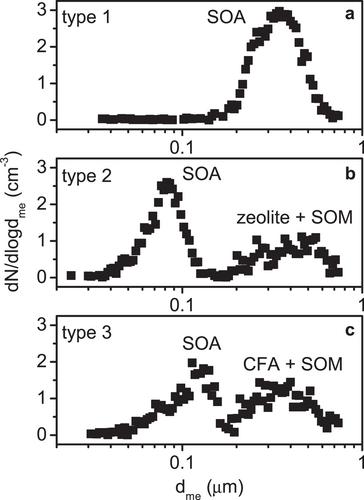
During processing in deep convective cloud systems, highly viscous or glassy secondary organic aerosol (SOA) particles can develop a porous structure through a process known as atmospheric freeze-drying. This structural modification may enhance their heterogeneous ice nucleation ability under cirrus conditions through the pore condensation and freezing mechanism. Pristine, compact SOA particles, on the other hand, are recommended to be treated as ice-inactive in models. This recommendation also applies to internally mixed particles, where a coating layer of secondary organic matter (SOM) deactivates the intrinsic ice nucleation ability of the core, which may be a mineral dust grain. Ice cloud-processing may also improve the ice nucleation ability of such a composite particle by inducing structural changes in the coating layer, which can release active sites on the mineral surface. In this work, we investigated the change in the ice nucleation ability of pure SOA particles from the ozonolysis of α-pinene and two types of internally mixed particles (zeolite and coal fly ash particles coated with SOM) after being subjected to the atmospheric freeze-drying process simulated in an expansion cloud chamber. For pure α-pinene SOA, we found only a slight improvement in the ice nucleation ability of the ice cloud-processed, porous particles compared to their pristine, compact counterparts at 221 and 217 K. In contrast, the zeolite and coal fly ash particles, which were initially deactivated by the organic coating, became significantly more ice-active after atmospheric freeze-drying, emphasizing that such composite particles cannot be excluded from model simulations of heterogeneous ice formation.