Immune checkpoint inhibitors (ICIs) are becoming more frequently used in the treatment of many types of malignant cancers by disinhibiting T-cell activation, which promotes the destruction of cancer cells. This disinhibition can also result in autoimmune conditions, like endocrinopathies.
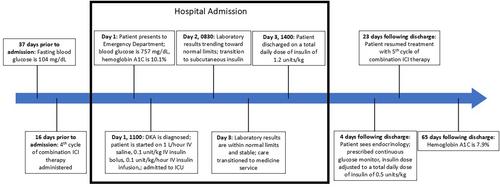
We report a case of a 78-year-old male patient with malignant mesothelioma treated with combination ICI therapy who presented with diabetic ketoacidosis (DKA) with no history of diabetes mellitus or hyperglycemia. The patient was admitted to the intensive care unit and treated with intravenous (IV) fluid repletion and IV insulin for DKA. The patient was diagnosed with new-onset type 1 diabetes mellitus (T1DM) induced by ICI therapy.
Approximately 75% of patients diagnosed with ICI-induced T1DM initially present with DKA. This, along with the rapid onset of hyperglycemia in this patient, suggests current guidelines for monitoring blood glucose are inadequate. Current guidelines recommend monitoring blood glucose at the following times: baseline, at the initiation of each cycle for 12 weeks, and then every 3–6 weeks thereafter. We propose the following schedule for monitoring blood glucose in patients receiving ICI therapy: baseline, twice weekly for the first six cycles, and then once weekly thereafter. This proposed update is supported by our patient's rapid onset of hyperglycemia and other case reports and reviews showing that most patients with this diagnosis have an initial presentation of DKA. Detecting hyperglycemia and starting treatment early is important in the prevention of acute complications from uncontrolled T1DM, like DKA.
This case adds to the existing body of literature and provides support for more frequent monitoring of blood glucose in patients receiving ICI therapy. Blood glucose monitoring is a simple, reliable, low risk, and inexpensive laboratory test that should be used in patients receiving ICI therapy to ensure prompt diagnosis and treatment of T1DM.