Elevated levels of CD11c+ myeloid cells are observed in various pulmonary disorders, including Idiopathic Pulmonary Fibrosis (IPF). Dendritic cells (DCs) and macrophages (MΦ) are critical antigen-presenting cells (APCs) that direct adaptive immunity. However, the role of CD11c+ myeloid cells in lung extracellular matrix (ECM) accumulation and pulmonary fibrosis is poorly understood.
We aimed to investigate the impact of depleting CD11c+ myeloid cells, including DCs and macrophages, during bleomycin-induced pulmonary fibrosis in mice.
We used a diphtheria toxin (DTx) receptor (DTR) transgenic mouse model (CD11c-DTR-Tg) to deplete CD11c+ myeloid cells through two methods: Systemic Depletion (SD) via intraperitoneal injection (i.p.) and local depletion (LD) via intranasal instillation (i.n.). We then assessed the effects of CD11c+ cell depletion during bleomycin-induced lung inflammation and fibrosis.
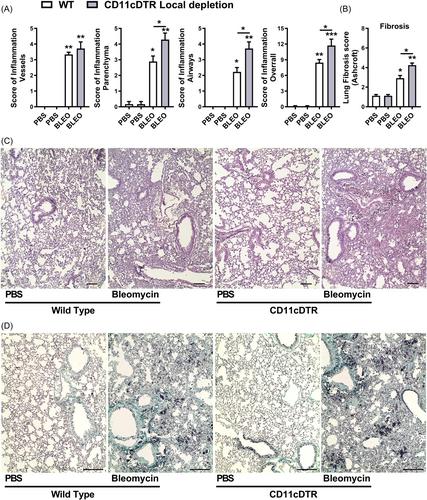
Fourteen days after bleomycin instillation, there was a progressive accumulation of myeloid cells, specifically F4/80-MHCII+CD11c+ DCs and F4/80 + MHCII+CD11c+ MΦ, preceding mortality and pulmonary fibrosis. Systemic depletion of CD11c+ DCs and MΦ via i.p. DTx administration in CD11c-DTR-Tg mice protected against bleomycin-induced mortality and pulmonary fibrosis compared to wild-type (WT) mice. Systemic depletion reduced myeloid cells, airway inflammation (total leukocytes, neutrophils, and CD4+ lymphocytes in bronchoalveolar lavage (BAL), inflammatory and fibrogenic mediators, and fibrosis-related mRNAs (Collagen-1α1 and α-SMA). Increased anti-inflammatory cytokine IL-10 and CXCL9 levels were observed, resulting in lower lung hydroxyproline content and Ashcroft fibrosis score. Conversely, local depletion of CD11c+ cells increased mortality by acute leukocyte influx (predominantly neutrophils, DCs, and MΦ in BAL) correlated to IL-1β, with lung hyper-inflammation and early fibrosis development.
Systemic depletion of CD11c+ cells confers protection against inflammation and fibrosis induced by Bleomycin, underscoring the significance of myeloid cells expressing F4/80-MHCII+CD11c+ DCs and F4/80 + MHCII+CD11c+ MΦ orchestrating the inflammatory milieu within the lungs, potentially as a source of cytokines sustaining pulmonary chronic inflammation leading to progressive fibrosis and mortality.