Exclusive enteral nutrition (EEN) is an established dietary therapy for Crohn's disease but its role in ulcerative colitis remains unclear.
To investigate the efficacy of EEN in adults with active ulcerative colitis and compare variations in treatment protocols, safety, tolerability and adherence.
We conducted a systematic search of MEDLINE, Embase, Cochrane CENTRAL, Emcare, CINAHL, Web of Science and trial registries for articles published from inception until July 21, 2024. We included all experimental and observational studies that described the use of EEN in adults with active ulcerative colitis. This review was registered on PROSPERO (CRD42022319584).
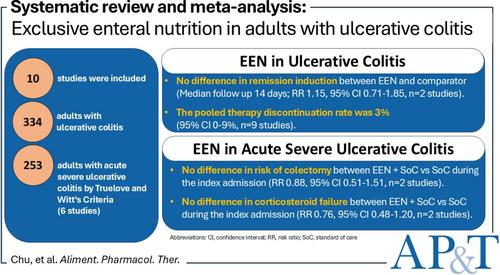
Of 3273 articles screened, we included 10 studies (334 adults). Overall, there was no difference between EEN and comparator for ulcerative colitis remission induction (median follow-up 14 days, risk ratio (RR) 1.15, 95% confidence interval (CI) 0.71–1.85; 2 studies). In acute severe ulcerative colitis, there was no difference between EEN and comparator for corticosteroid failure (RR 0.76, 95% CI 0.48–1.20; 2 studies) or risk of colectomy (RR 0.88, 95% CI 0.51–1.51, n = 2 studies) during index admission. The pooled discontinuation rate was 3% (95% CI 0–10; 9 studies). There was heterogeneity in trial design, methodology and assessment of outcomes.
EEN was well tolerated with low therapy discontinuation in adults with active ulcerative colitis. However, there is insufficient evidence to support the use of EEN as an adjunctive therapy to standard of care. Further, well-designed studies with reproducible methodology and endpoints are necessary to evaluate its effectiveness.
PROSPERO 2022 CRD42022319584.