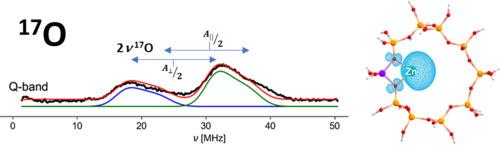
Oxide-based materials are of key technological importance in different areas including advanced functional materials, solid state chemistry and catalysis. Many of the key questions concerning these areas involve understanding the chemical bond between the metal and the oxygen ions in the first or subsequent coordinating shells. The spectroscopic study of oxygen is therefore of fundamental importance to elucidate the complex interfacial coordination chemistry that underlies the development of metal-oxide supported catalysts and other advanced materials. Oxygen atoms at solid surfaces or lining the pores of zeolite frameworks play a vital role in stabilizing and defining the electronic and geometric structure of single metal atoms or clusters that act as catalytically active sites. In the case of paramagnetic species, EPR and its related hyperfine techniques offer a unique opportunity to explore and understand the nature of the chemical bonding in metal-oxide systems through the detection of the 17O hyperfine interaction. In this perspective we offer an overview of experimental considerations and relevant examples specific to 17O hyperfine spectroscopy of transition metal ions in zeolites relevant to catalysis. 17O hyperfine coupling values are obtained, which allow discriminating σ- and π-bonding channels in metal-oxygen bonds involving first-row transition metal ions. An exhaustive collection of 17O hyperfine and nuclear quadrupole couplings in different systems including molecular and biomolecular chemistry is provided, emphasizing the connection between interfacial and molecular inorganic coordination chemistry.