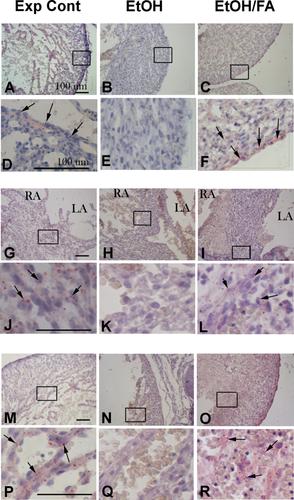
{"title":"Acute alcohol exposure during mouse gastrulation alters lipid metabolism in placental and heart development: Folate prevention","authors":"Kersti K. Linask, Mingda Han","doi":"10.1002/bdra.23526","DOIUrl":null,"url":null,"abstract":"Background Embryonic acute exposure to ethanol (EtOH), lithium, and homocysteine (HCy) induces cardiac defects at the time of exposure; folic acid (FA) supplementation protects normal cardiogenesis (Han et al., 2009, 2012; Serrano et al., 2010). Our hypothesis is that EtOH exposure and FA protection relate to lipid and FA metabolism during mouse cardiogenesis and placentation. Methods On the morning of conception, pregnant C57BL/6J mice were placed on either of two FA‐containing diets: a 3.3 mg health maintenance diet or a high FA diet of 10.5 mg/kg. Mice were injected a binge level of EtOH, HCy, or saline on embryonic day (E) 6.75, targeting gastrulation. On E15.5, cardiac and umbilical blood flow were examined by ultrasound. Embryonic cardiac tissues were processed for gene expression of lipid and FA metabolism; the placenta and heart tissues for neutral lipid droplets, or for medium chain acyl‐dehydrogenase (MCAD) protein. Results EtOH exposure altered lipid‐related gene expression on E7.5 in comparison to control or FA‐supplemented groups and remained altered on E15.5 similarly to changes with HCy, signifying FA deficiency. In comparison to control tissues, the lipid‐related acyl CoA dehydrogenase medium length chain gene and its protein MCAD were altered with EtOH exposure, as were neutral lipid droplet localization in the heart and placenta. Conclusion EtOH altered gene expression associated with lipid and folate metabolism, as well as neutral lipids, in the E15.5 abnormally functioning heart and placenta. In comparison to controls, the high FA diet protected the embryo and placenta from these effects allowing normal development. Birth Defects Research (Part A) 106:749–760, 2016. © 2016 The Authors Birth Defects Research Part A: Clinical and Molecular Teratology Published by Wiley Periodicals, Inc.","PeriodicalId":8983,"journal":{"name":"Birth defects research. Part A, Clinical and molecular teratology","volume":"106 9","pages":"749-760"},"PeriodicalIF":0.0000,"publicationDate":"2016-06-14","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/bdra.23526","citationCount":"16","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Birth defects research. Part A, Clinical and molecular teratology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/bdra.23526","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q","JCRName":"Medicine","Score":null,"Total":0}
引用次数: 16
Abstract
Background Embryonic acute exposure to ethanol (EtOH), lithium, and homocysteine (HCy) induces cardiac defects at the time of exposure; folic acid (FA) supplementation protects normal cardiogenesis (Han et al., 2009, 2012; Serrano et al., 2010). Our hypothesis is that EtOH exposure and FA protection relate to lipid and FA metabolism during mouse cardiogenesis and placentation. Methods On the morning of conception, pregnant C57BL/6J mice were placed on either of two FA‐containing diets: a 3.3 mg health maintenance diet or a high FA diet of 10.5 mg/kg. Mice were injected a binge level of EtOH, HCy, or saline on embryonic day (E) 6.75, targeting gastrulation. On E15.5, cardiac and umbilical blood flow were examined by ultrasound. Embryonic cardiac tissues were processed for gene expression of lipid and FA metabolism; the placenta and heart tissues for neutral lipid droplets, or for medium chain acyl‐dehydrogenase (MCAD) protein. Results EtOH exposure altered lipid‐related gene expression on E7.5 in comparison to control or FA‐supplemented groups and remained altered on E15.5 similarly to changes with HCy, signifying FA deficiency. In comparison to control tissues, the lipid‐related acyl CoA dehydrogenase medium length chain gene and its protein MCAD were altered with EtOH exposure, as were neutral lipid droplet localization in the heart and placenta. Conclusion EtOH altered gene expression associated with lipid and folate metabolism, as well as neutral lipids, in the E15.5 abnormally functioning heart and placenta. In comparison to controls, the high FA diet protected the embryo and placenta from these effects allowing normal development. Birth Defects Research (Part A) 106:749–760, 2016. © 2016 The Authors Birth Defects Research Part A: Clinical and Molecular Teratology Published by Wiley Periodicals, Inc.
小鼠原肠胚期急性酒精暴露改变胎盘和心脏发育中的脂质代谢:叶酸预防
胚胎急性暴露于乙醇(EtOH)、锂和同型半胱氨酸(HCy)时可诱发心脏缺陷;补充叶酸(FA)可以保护正常的心脏发生(Han et al., 2009, 2012;Serrano et al., 2010)。我们的假设是,EtOH暴露和FA保护与小鼠心脏发生和胎盘过程中的脂质和FA代谢有关。方法在受孕当天早晨,将妊娠C57BL/6J小鼠分别饲喂3.3 mg健康维持饲粮和10.5 mg/kg高FA饲粮。小鼠在胚胎日(E) 6.75狂饮注射EtOH、HCy或生理盐水,目标是原肠胚形成。在E15.5,用超声检查心脏和脐带血流量。对胚胎心脏组织进行脂质和FA代谢基因表达分析;胎盘和心脏组织的中性脂滴,或中链酰基脱氢酶(MCAD)蛋白。结果与对照组或FA补充组相比,EtOH暴露改变了E7.5上脂质相关基因的表达,并且在E15.5上保持不变,与HCy相似,表明FA缺乏。与对照组织相比,脂肪相关的酰基辅酶a脱氢酶中长链基因及其蛋白MCAD随着EtOH暴露而改变,心脏和胎盘中的中性脂滴定位也发生了变化。结论EtOH改变了E15.5异常心脏和胎盘中与脂质和叶酸代谢以及中性脂质的相关基因表达。与对照组相比,高FA饮食保护胚胎和胎盘免受这些影响,使其正常发育。出生缺陷研究(A辑)(06):749 - 760,2016。©2016 The Authors Birth Defects Research Part A: Clinical and Molecular Teratology by Wiley journals, Inc.出版
本文章由计算机程序翻译,如有差异,请以英文原文为准。



