{"title":"Discovery of Electrophiles and Profiling of Enzyme Cofactors","authors":"Suzanne E. Dettling, Mina Ahmadi, Zongtao Lin, Lin He, Megan L. Matthews","doi":"10.1002/cpch.86","DOIUrl":null,"url":null,"abstract":"<p>Reverse-polarity activity-based protein profiling (RP-ABPP) is a chemical proteomics approach that uses nucleophilic probes amenable to “click” chemistry deployed into living cells in culture to capture, immunoprecipitate, and identify protein-bound electrophiles. RP-ABPP is used to characterize the structure and function of reactive electrophilic post-translational modifications (PTMs) and the proteins harboring them, which may uncover unknown or novel functions. RP-ABPP has demonstrated utility as a versatile method to monitor the metabolic regulation of electrophilic cofactors, using a pyruvoyl cofactor in <i>S</i>-adenosyl-<span>L</span>-methionine decarboxylase (AMD1), and to discover novel types of electrophilic modifications on proteins in human cells, such as the glyoxylyl modification on secernin-3 (SCRN3). These cofactors cannot be predicted by sequence, and therefore this area is relatively undeveloped. RP-ABPP is the only global, unbiased approach to discover such electrophiles. Here, we describe the utility of these experiments and provide a detailed protocol for de novo discovery, quantitation, and global profiling of electrophilic functionality of proteins. © 2020 The Authors.</p><p><b>Basic Protocol 1</b>: Identification and quantification of probe-reactive proteins</p><p><b>Basic Protocol 2</b>: Characterization of the site of probe labeling</p><p><b>Basic Protocol 3</b>: Determination and quantitation of electrophile structure</p>","PeriodicalId":38051,"journal":{"name":"Current protocols in chemical biology","volume":"12 4","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2020-11-16","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cpch.86","citationCount":"8","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols in chemical biology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpch.86","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q3","JCRName":"Biochemistry, Genetics and Molecular Biology","Score":null,"Total":0}
引用次数: 8
Abstract
Reverse-polarity activity-based protein profiling (RP-ABPP) is a chemical proteomics approach that uses nucleophilic probes amenable to “click” chemistry deployed into living cells in culture to capture, immunoprecipitate, and identify protein-bound electrophiles. RP-ABPP is used to characterize the structure and function of reactive electrophilic post-translational modifications (PTMs) and the proteins harboring them, which may uncover unknown or novel functions. RP-ABPP has demonstrated utility as a versatile method to monitor the metabolic regulation of electrophilic cofactors, using a pyruvoyl cofactor in S-adenosyl-L-methionine decarboxylase (AMD1), and to discover novel types of electrophilic modifications on proteins in human cells, such as the glyoxylyl modification on secernin-3 (SCRN3). These cofactors cannot be predicted by sequence, and therefore this area is relatively undeveloped. RP-ABPP is the only global, unbiased approach to discover such electrophiles. Here, we describe the utility of these experiments and provide a detailed protocol for de novo discovery, quantitation, and global profiling of electrophilic functionality of proteins. © 2020 The Authors.
Basic Protocol 1: Identification and quantification of probe-reactive proteins
Basic Protocol 2: Characterization of the site of probe labeling
Basic Protocol 3: Determination and quantitation of electrophile structure
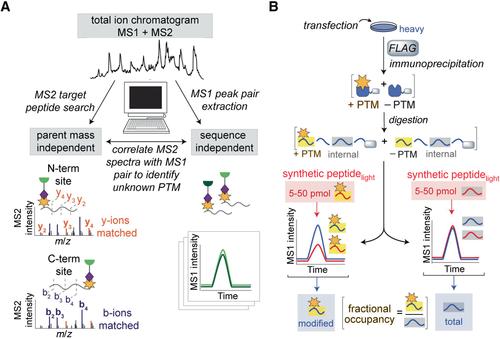
亲电试剂的发现和酶辅助因子的分析
基于反极性活性的蛋白质分析(RP-ABPP)是一种化学蛋白质组学方法,它使用亲核探针,可“点击”化学部署到培养的活细胞中,以捕获,免疫沉淀和识别蛋白质结合的亲电试剂。RP-ABPP用于表征反应性亲电翻译后修饰(PTMs)及其蛋白的结构和功能,这可能揭示未知或新的功能。RP-ABPP已被证明是一种多功能的方法,用于监测亲电辅助因子的代谢调节,利用s -腺苷- l-甲硫氨酸脱羧酶(AMD1)中的丙酮酰辅助因子,并发现人类细胞中蛋白质的新型亲电修饰,如丝氨酸-3 (SCRN3)上的乙基酰修饰。这些辅因子不能通过序列预测,因此该地区相对不发达。RP-ABPP是发现此类亲电试剂的唯一全球性、公正的方法。在这里,我们描述了这些实验的效用,并为蛋白质亲电功能的从头发现、定量和全局分析提供了详细的方案。©2020作者。基本方案1:探针反应蛋白的鉴定和定量基本方案2:探针标记位点的表征基本方案3:亲电试剂结构的测定和定量
本文章由计算机程序翻译,如有差异,请以英文原文为准。



