Measuring Mitochondrial Respiration in Previously Frozen Biological Samples
Corey Osto, Ilan Y. Benador, Jennifer Ngo, Marc Liesa, Linsey Stiles, Rebeca Acin-Perez, Orian S. Shirihai
下载PDF
{"title":"Measuring Mitochondrial Respiration in Previously Frozen Biological Samples","authors":"Corey Osto, Ilan Y. Benador, Jennifer Ngo, Marc Liesa, Linsey Stiles, Rebeca Acin-Perez, Orian S. Shirihai","doi":"10.1002/cpcb.116","DOIUrl":null,"url":null,"abstract":"<p>Measuring oxygen consumption allows for the role of mitochondrial function in biological phenomena and mitochondrial diseases to be determined. Although respirometry has become a common approach in disease research, current methods are limited by the necessity to process and measure tissue samples within 1 hr of acquisition. Detailed by Acin-Perez and colleagues, a new respirometry approach designed for previously frozen tissue samples eliminates these hurdles for mitochondrial study. This technique allows for the measurement of maximal respiratory capacity in samples frozen for long-term storage before testing. This protocol article describes the optimal tissue isolation methods and the combination of substrates to define electron transport chain function at high resolution in previously frozen tissue samples. © 2020 The Authors.</p><p><b>Basic Protocol 1</b>: Sample collection, storage, and homogenization for previously frozen tissue respirometry</p><p><b>Basic Protocol 2</b>: Running a Seahorse respirometry assay using previously frozen tissue samples</p><p><b>Basic Protocol 3</b>: Normalization to mitochondrial content for previously frozen tissue respirometry</p>","PeriodicalId":40051,"journal":{"name":"Current Protocols in Cell Biology","volume":"89 1","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2020-12-15","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cpcb.116","citationCount":"26","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current Protocols in Cell Biology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpcb.116","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q3","JCRName":"Biochemistry, Genetics and Molecular Biology","Score":null,"Total":0}
引用次数: 26
引用
批量引用
Abstract
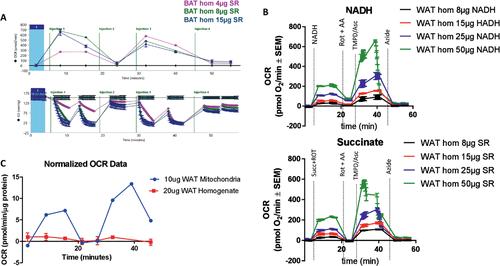
Measuring oxygen consumption allows for the role of mitochondrial function in biological phenomena and mitochondrial diseases to be determined. Although respirometry has become a common approach in disease research, current methods are limited by the necessity to process and measure tissue samples within 1 hr of acquisition. Detailed by Acin-Perez and colleagues, a new respirometry approach designed for previously frozen tissue samples eliminates these hurdles for mitochondrial study. This technique allows for the measurement of maximal respiratory capacity in samples frozen for long-term storage before testing. This protocol article describes the optimal tissue isolation methods and the combination of substrates to define electron transport chain function at high resolution in previously frozen tissue samples. © 2020 The Authors.
Basic Protocol 1 : Sample collection, storage, and homogenization for previously frozen tissue respirometry
Basic Protocol 2 : Running a Seahorse respirometry assay using previously frozen tissue samples
Basic Protocol 3 : Normalization to mitochondrial content for previously frozen tissue respirometry
测定先前冷冻生物样品中的线粒体呼吸作用
测量氧气消耗可以确定线粒体功能在生物现象和线粒体疾病中的作用。虽然呼吸测量已成为疾病研究中的常用方法,但目前的方法受到需要在获取组织样本后1小时内处理和测量组织样本的限制。Acin-Perez及其同事详细介绍了一种新的呼吸测量方法,该方法专为先前冷冻的组织样本设计,消除了线粒体研究的这些障碍。该技术允许在测试前测量长期冷冻储存的样品的最大呼吸能力。本协议文章描述了最佳的组织分离方法和底物的组合,以确定电子传递链功能在高分辨率先前冷冻组织样品。©2020作者。基本方案1:先前冷冻组织呼吸测定的样本收集、储存和均质基本方案2:使用先前冷冻组织样本进行海马呼吸测定基本方案3:先前冷冻组织呼吸测定的线粒体含量归一化
本文章由计算机程序翻译,如有差异,请以英文原文为准。