This study aims to compare the real-world effectiveness and safety of Endostar in combination with chemotherapy in the treatment of advanced non-small cell lung cancer (NSCLC) in different age groups.
Electronic medical records of patients with NSCLC who received Endostar combined with chemotherapy from June 2012 to August 2019 in 7 cancer centers were retrospectively collected. Baseline characteristics before and after propensity score matching (PSM), effectiveness evaluation, and safety data of two age groups were analyzed. Tumor response was evaluated according to RECIST v1.1. Adverse events (AEs) were graded according to NCI-CTCAE 5.0.
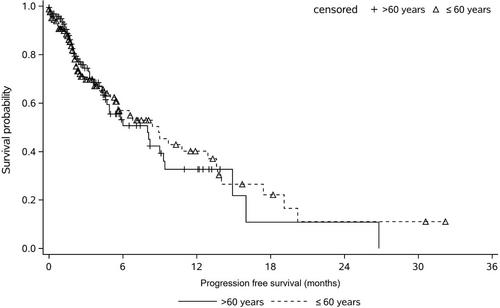
In this study, 554 and 571 patients were assigned to ≤60 years non-aged group and >60 years aged group, respectively. After propensity score matching (PSM) was introduced, 166 patients in each age group were analyzed. The median PFS for the two groups was 8.9 and 8.0 months, with the overall response rate was 24.7% and 26.5% (p = 0.7060), disease control rate was 64.5% versus 68.7% (p = 0.4600), respectively. Cox regression result showed that advanced age has no significant influence on PFS (hazard ratio = 1.02, 95% CI: 0.98−1.06, p = 0.3034) in contrast with younger patients. The most common AEs in both age groups were myelosuppression, gastrointestinal reactions, and hepatic impairment. The total incidence for the above AEs in the two groups was 18.67% versus 24.10%, 22.89% versus 21.69%, 12.05% versus 7.23%, with no statistically significant difference.
Compared with treating patients with NSCLC younger than 60 years old, the effectiveness of Endostar combined with chemotherapy in treating advanced patients showed no significant differences, with tolerable adverse events.