P7C3 Ameliorates Bone Loss by Inhibiting Osteoclast Differentiation and Promoting Osteogenesis
Bo Tian, Jinyu Bai, Lei Sheng, Hao Chen, Wenju Chang, Yue Zhang, Chenlu Yao, Chenmeng Zhou, Xiaoyu Wang, Huajian Shan, Qirong Dong, Chao Wang, Xiaozhong Zhou
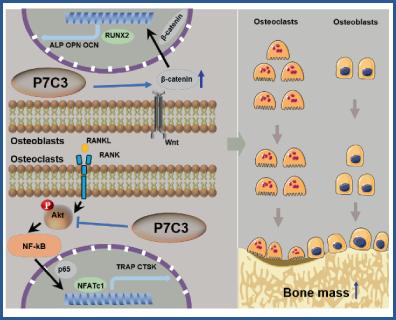
{"title":"P7C3 Ameliorates Bone Loss by Inhibiting Osteoclast Differentiation and Promoting Osteogenesis","authors":"Bo Tian, Jinyu Bai, Lei Sheng, Hao Chen, Wenju Chang, Yue Zhang, Chenlu Yao, Chenmeng Zhou, Xiaoyu Wang, Huajian Shan, Qirong Dong, Chao Wang, Xiaozhong Zhou","doi":"10.1002/jbm4.10811","DOIUrl":null,"url":null,"abstract":"<p>Bone homeostasis, the equilibrium between bone resorption and formation, is essential for maintaining healthy bone tissue in adult humans. Disruptions of this process can lead to pathological conditions such as osteoporosis. Dual-targeted agents, capable of inhibiting excessive bone resorption and stimulating bone formation, are being explored as a promising strategy for developing new treatments to address osteoporosis. In this study, we investigated the effects of P7C3 on bone remodeling and its potential therapeutic role in osteoporosis treatment in mice. Specifically, P7C3 can remarkably suppress receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL)-induced osteoclast differentiation in bone marrow macrophages via the Akt-NF-κB-NFATc1 signaling pathway. Additionally, RNA sequencing (RNAseq) analysis revealed that P7C3 promoted osteoblast differentiation and function through the Wnt/β-catenin signaling pathway, thereby enhancing bone formation. Furthermore, μCT analysis and histological examination of bone tissues from P7C3-treated mice showed attenuation of both Ti-induced bone erosion and ovariectomy (OVX)-induced bone loss. These findings suggest that P7C3 may have a novel function in bone remodeling and may be a promising therapeutic agent for the treatment of osteoporosis. © 2023 The Authors. <i>JBMR Plus</i> published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.</p>","PeriodicalId":14611,"journal":{"name":"JBMR Plus","volume":"7 12","pages":""},"PeriodicalIF":2.4000,"publicationDate":"2023-09-06","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://asbmr.onlinelibrary.wiley.com/doi/epdf/10.1002/jbm4.10811","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"JBMR Plus","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jbm4.10811","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"ENDOCRINOLOGY & METABOLISM","Score":null,"Total":0}
引用次数: 0
Abstract
Bone homeostasis, the equilibrium between bone resorption and formation, is essential for maintaining healthy bone tissue in adult humans. Disruptions of this process can lead to pathological conditions such as osteoporosis. Dual-targeted agents, capable of inhibiting excessive bone resorption and stimulating bone formation, are being explored as a promising strategy for developing new treatments to address osteoporosis. In this study, we investigated the effects of P7C3 on bone remodeling and its potential therapeutic role in osteoporosis treatment in mice. Specifically, P7C3 can remarkably suppress receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL)-induced osteoclast differentiation in bone marrow macrophages via the Akt-NF-κB-NFATc1 signaling pathway. Additionally, RNA sequencing (RNAseq) analysis revealed that P7C3 promoted osteoblast differentiation and function through the Wnt/β-catenin signaling pathway, thereby enhancing bone formation. Furthermore, μCT analysis and histological examination of bone tissues from P7C3-treated mice showed attenuation of both Ti-induced bone erosion and ovariectomy (OVX)-induced bone loss. These findings suggest that P7C3 may have a novel function in bone remodeling and may be a promising therapeutic agent for the treatment of osteoporosis. © 2023 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.