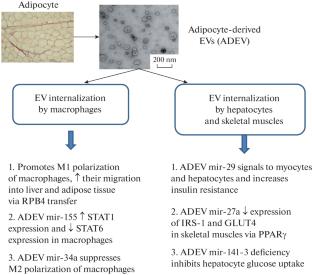
Extracellular vesicles (EVs) are spherical functionally important structures of cell membrane origin ranging in size from 40 nm to 5000 nm. They are involved in the horizontal transfer of mainly proteins and microRNAs. The mechanisms of EV internalization include clathrin-dependent endocytosis, caveolin-dependent endocytosis, raft-mediated endocytosis, and macropinocytosis. Type 2 diabetes mellitus (T2DM) is a common group of metabolic disorders in adults; the incidence and prevalence of T2DM increase in parallel with the obesity epidemic. Since adipose tissue plays a key role in the development of insulin resistance, EVs secreted by adipose tissue may be considered as an information transmitter in this process. EVs of the adipocyte origin are predominantly captured up by tissue macrophages, adipocytes themselves, hepatocytes, and skeletal muscles. The EV uptake promotes M1 polarization of macrophages, a decrease in glucose uptake by hepatocytes and myocytes due to the transfer of functionally active microRNAs influencing carbohydrate and lipid metabolism. Patients with T2DM and impaired glucose tolerance, have a significantly higher level of CD235a-positive (erythrocyte) EVs and a trend to the increase in CD68-positive (leukocyte) and CD62p-positive (platelet/endothelial cells) EVs. The levels of CD31+/CD146-positive EVs (endothelial cells) were comparable between diabetic and euglycemic patients. EVs from diabetic patients were preferentially internalized by monocytes (predominantly classical and transitional, and to a lesser extent nonclassical monocyte fractions) and B cells as compared to euglycemic patients. Internalization of EVs from patients with T2DM by monocytes led to a decrease in their apoptosis, changes in differentiation, and suppression of monocyte reactions controlling oxidative stress. Thus, insulin resistance increases the secretion of EVs, which are preferentially internalized by monocytes and alter their function. EVs are considered as sources of promising clinical markers of insulin resistance, complications of diabetes mellitus (endothelial dysfunction, retinopathy, nephropathy, neuropathy), and EV markers can also be used to monitor the effectiveness of therapy for these complications.