下载PDF
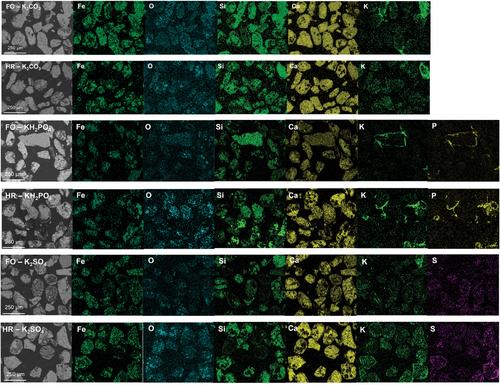
{"title":"Interactions between potassium ashes and oxygen carriers based on natural and waste materials at different initial oxidation states","authors":"Victor Purnomo, Fredrik Hildor, Pavleta Knutsson, Henrik Leion","doi":"10.1002/ghg.2208","DOIUrl":null,"url":null,"abstract":"<p>One of the most essential features of an oxygen carrier is its ability to be oxidized and reduced in order to transfer oxygen in a chemical looping system. A highly reduced oxygen carrier can experience multiple performance issues, such as decreased reactivity, agglomeration, and defluidization. This is crucial for processes that require limited oxygen transfer from the air reactor to the fuel reactor. Meanwhile, biomasses as environmentally friendly fuel options contain ashes, which would inevitably react with oxygen carriers and exacerbate the performance issues. To mimic the interactions between a highly reduced oxygen carrier and biomass ash compounds, four iron-based oxygen carriers, based on natural ores and waste materials, and three potassium salts, K<sub>2</sub>CO<sub>3</sub>, KH<sub>2</sub>PO<sub>4</sub>, and K<sub>2</sub>SO<sub>4</sub>, were investigated in a tubular reactor under an atmosphere consisting of 2.5% H<sub>2</sub> and 10% steam in Ar and N<sub>2</sub> at 900°C for 3 h. The results from the X-ray diffraction (XRD) material analysis showed that both initially fully oxidized and highly reduced materials reach the same oxidation state after the experiment. Based on the scanning electron microscopy coupled with energy dispersive X-ray spectroscopy results, K from K<sub>2</sub>CO<sub>3</sub> and K<sub>2</sub>SO<sub>4</sub> diffuses in the oxygen carrier particles, while K from KH<sub>2</sub>PO<sub>4</sub> always forms a distinct layer around the particles. The initial oxidation state of an oxygen carrier surface affects the interactions with the potassium salt only to minor extents. Thus, the final state of the material and its performance in a large-scale process are only occasionally and mildly affected by its initial oxidation state. © 2023 The Authors. <i>Greenhouse Gases: Science and Technology</i> published by Society of Chemical Industry and John Wiley & Sons Ltd.</p>","PeriodicalId":12796,"journal":{"name":"Greenhouse Gases: Science and Technology","volume":"13 4","pages":"520-534"},"PeriodicalIF":2.7000,"publicationDate":"2023-03-11","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/ghg.2208","citationCount":"1","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Greenhouse Gases: Science and Technology","FirstCategoryId":"93","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/ghg.2208","RegionNum":4,"RegionCategory":"环境科学与生态学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q3","JCRName":"ENERGY & FUELS","Score":null,"Total":0}
引用次数: 1
引用
批量引用
Abstract
One of the most essential features of an oxygen carrier is its ability to be oxidized and reduced in order to transfer oxygen in a chemical looping system. A highly reduced oxygen carrier can experience multiple performance issues, such as decreased reactivity, agglomeration, and defluidization. This is crucial for processes that require limited oxygen transfer from the air reactor to the fuel reactor. Meanwhile, biomasses as environmentally friendly fuel options contain ashes, which would inevitably react with oxygen carriers and exacerbate the performance issues. To mimic the interactions between a highly reduced oxygen carrier and biomass ash compounds, four iron-based oxygen carriers, based on natural ores and waste materials, and three potassium salts, K2 CO3 , KH2 PO4 , and K2 SO4 , were investigated in a tubular reactor under an atmosphere consisting of 2.5% H2 and 10% steam in Ar and N2 at 900°C for 3 h. The results from the X-ray diffraction (XRD) material analysis showed that both initially fully oxidized and highly reduced materials reach the same oxidation state after the experiment. Based on the scanning electron microscopy coupled with energy dispersive X-ray spectroscopy results, K from K2 CO3 and K2 SO4 diffuses in the oxygen carrier particles, while K from KH2 PO4 always forms a distinct layer around the particles. The initial oxidation state of an oxygen carrier surface affects the interactions with the potassium salt only to minor extents. Thus, the final state of the material and its performance in a large-scale process are only occasionally and mildly affected by its initial oxidation state. © 2023 The Authors. Greenhouse Gases: Science and Technology published by Society of Chemical Industry and John Wiley & Sons Ltd.
基于不同初始氧化态的天然和废料的钾灰与氧载体之间的相互作用
氧载体最基本的特征之一是它能够被氧化和还原,以便在化学环系统中传递氧。高度还原的氧载体会遇到多种性能问题,如反应性降低、团聚和脱流。这对于需要从空气反应堆到燃料反应堆的有限氧气传输的过程至关重要。同时,作为环保燃料的生物质含有灰烬,不可避免地会与氧载体发生反应,从而加剧性能问题。为了模拟高度还原的氧载体与生物质灰化合物、四种基于天然矿石和废料的铁基氧载体以及三种钾盐(K2CO3、KH2PO4和K2SO4)之间的相互作用,在管式反应器中,在由2.5% H2和10%水蒸气组成的氩气和N2气氛中,900℃下反应3 h。x射线衍射(XRD)材料分析结果表明,经过实验,初始完全氧化和高度还原的材料都达到了相同的氧化状态。扫描电镜结合能量色散x射线能谱分析结果表明,K2CO3和K2SO4中的K在载氧粒子中扩散,而KH2PO4中的K在载氧粒子周围总是形成明显的层。氧载体表面的初始氧化态对其与钾盐的相互作用影响不大。因此,在大规模工艺过程中,材料的最终状态及其性能只偶尔和轻微地受到其初始氧化态的影响。©2023作者。温室气体:科学与技术,化学工业学会和约翰·威利出版社出版;子有限公司
本文章由计算机程序翻译,如有差异,请以英文原文为准。