Preparation of Primary Rat Hepatocyte Spheroids Utilizing the Liquid-Overlay Technique
Jonathan A. Kyffin, Christopher R. Cox, Joseph Leedale, Helen E. Colley, Craig Murdoch, Pratibha Mistry, Steven D. Webb, Parveen Sharma
{"title":"Preparation of Primary Rat Hepatocyte Spheroids Utilizing the Liquid-Overlay Technique","authors":"Jonathan A. Kyffin, Christopher R. Cox, Joseph Leedale, Helen E. Colley, Craig Murdoch, Pratibha Mistry, Steven D. Webb, Parveen Sharma","doi":"10.1002/cptx.87","DOIUrl":null,"url":null,"abstract":"<p>Herein, we describe a protocol for the preparation and analysis of primary isolated rat hepatocytes in a 3D cell culture format described as spheroids. The hepatocyte cells spontaneously self-aggregate into spheroids without the need for synthetic extracellular matrices or hydrogels. Primary rat hepatocytes (PRHs) are a readily available source of primary differentiated liver cells and therefore conserve many of the required liver-specific functional markers, and elicit the natural in vivo phenotype when compared with common hepatic cells lines. We describe the liquid-overlay technique which provides an ultra-low attachment surface on which PRHs can be cultured as spheroids. © 2019 The Authors.</p><p><b>Basic Protocol 1</b>: Preparation of agarose-coated plates</p><p><b>Basic Protocol 2</b>: Primary rat hepatocyte isolation procedure</p><p><b>Basic Protocol 3</b>: Primary rat hepatocyte spheroid culture</p><p><b>Basic Protocol 4</b>: Immunofluorescent analysis of PRH spheroids</p>","PeriodicalId":72743,"journal":{"name":"Current protocols in toxicology","volume":"81 1","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2019-09-13","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cptx.87","citationCount":"6","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols in toxicology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cptx.87","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 6
Abstract
Herein, we describe a protocol for the preparation and analysis of primary isolated rat hepatocytes in a 3D cell culture format described as spheroids. The hepatocyte cells spontaneously self-aggregate into spheroids without the need for synthetic extracellular matrices or hydrogels. Primary rat hepatocytes (PRHs) are a readily available source of primary differentiated liver cells and therefore conserve many of the required liver-specific functional markers, and elicit the natural in vivo phenotype when compared with common hepatic cells lines. We describe the liquid-overlay technique which provides an ultra-low attachment surface on which PRHs can be cultured as spheroids. © 2019 The Authors.
Basic Protocol 1: Preparation of agarose-coated plates
Basic Protocol 2: Primary rat hepatocyte isolation procedure
Basic Protocol 3: Primary rat hepatocyte spheroid culture
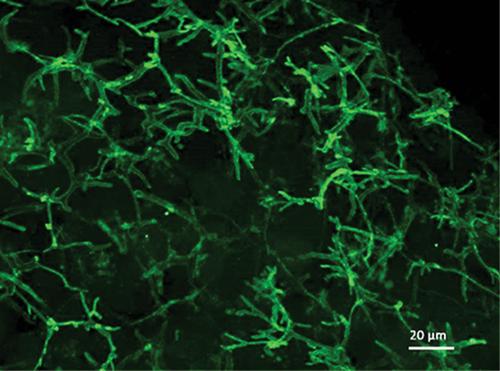
Basic Protocol 4: Immunofluorescent analysis of PRH spheroids
利用液体覆盖技术制备原代大鼠肝细胞球体
在这里,我们描述了一种方案,用于制备和分析原代分离的大鼠肝细胞在三维细胞培养格式描述为球体。肝细胞不需要合成细胞外基质或水凝胶就能自发聚集成球状体。原代大鼠肝细胞(PRHs)是一种容易获得的原代分化肝细胞来源,因此保留了许多所需的肝脏特异性功能标记,与普通肝细胞系相比,可引发自然的体内表型。我们描述了液体覆盖技术,该技术提供了一个超低附着表面,PRHs可以在其上作为球体培养。©2019作者。基本方案1:琼脂糖包被板的制备基本方案2:原代大鼠肝细胞分离程序基本方案3:原代大鼠肝细胞球体培养基本方案4:PRH球体的免疫荧光分析
本文章由计算机程序翻译,如有差异,请以英文原文为准。


