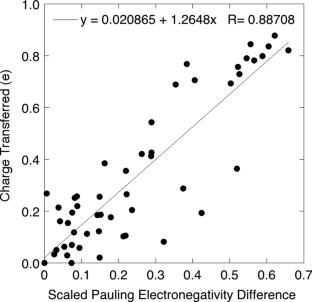
Formal charge and oxidation state are two means of estimating the charge of an atom in a molecule. Though these concepts have very different origins—formal charge is derived from the ball-and-hook model of bonding and oxidation state is based on the ionic approximation of molecules—they are used to predict reactivity and other molecular properties through their properties as charges. In this submission, it is shown that formal charge and oxidation state are two extreme descriptions of bonding: formal charge represents zero charge transfer between atoms while oxidation state represents complete charge transfer in each bond. These ‘localised electron approximations’ form an incomplete picture of atomic charge. Electronegativity measures the extent of polarity in real bonds; this concept can be introduced to polarise bonds relative to the ‘equal sharing model’. It is shown that the various electronegativity models are fundamentally related. We chose two models to demonstrate numerically that polar bonds yield charges intermediate between the localised electron approximations: Pauling and Mulliken. It is shown that probabilistic interpretation of the Pauling scale (‘scaled Pauling’ method) and use of asymmetric chemical potential (‘modified Mulliken’ method) lead to atomic charges that closely approximate experimental values using simple ‘back of the envelope’ calculations. It is seen that formal charge, oxidation state, and electronegativity-interpolated charge lie on a continuum and are mathematically related. It is therefore concluded that electronegativity introduces (quantum) delocalisation to the localised (classical) picture of electron bonding.