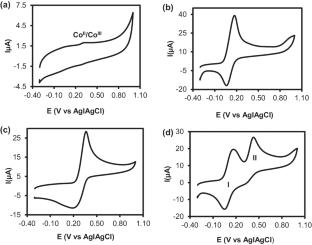
The fabrication of sensitive, fast, cost-effective and eco-friendly electrochemical sensors is essential for monitoring analytes of biomedical, environmental and pharmaceutical interests. Herein, we report the simultaneous electroreduction and deposition of graphene oxide (GO) to form electrochemically reduced graphene oxide (ERGO) onto a glassy carbon electrode (GCE) represented as GCE-ERGO. Onto the GCE-ERGO, cobalt (II) tetra-amino phthalocyanine was electropolymerized to form a stable GCE-ERGO/polyCoTAPc. The sensing surface, GCE-ERGO/polyCoTAPc, was characterized using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) techniques to ascertain its electron transfer and conducting properties. The modifier surface functional groups and composition were confirmed using infrared spectroscopy and energy-dispersive X-ray spectroscopy. The prepared sensing electrode displayed enhanced electrocatalytic activity towards ferri/ferrocyanide {[Fe(CN)6]3−/4−} as a redox probe. GCE-ERGO/polyCoTAPc was further used for ultrasensitive simultaneous detection and determination of dopamine (DA) and paracetamol (PA). The electrocatalytic peak currents for DA and PA were greatly enhanced with an oxidation potential difference of 264 mV, wide enough for simultaneous determination. Using differential pulse voltammetry (DPV), the electrocatalytic oxidation peak currents of DA and PA at GCE-ERGO/polyCoTAPc showed linear dependence with the changes in concentrations up to 100 µM for DA and up to 90 µM for PA. The limits of detection (LOD) values were 0.095 µM and 0.10 µM using a signal-to-noise (S/N) ratio of 3 for DA and PA, respectively. The GCE-ERGO/polyCoTAPc displayed excellent sensitivity of 8.39 µA µM−1 cm−2 for DA and 1.32 µA µM−1 cm−2 for PA. The fabricated ultrasensitive electrochemical sensor was successfully used for the determination of DA and PA in synthetic urine samples with excellent percentage recoveries.