下载PDF
{"title":"Polyelectrolytes with sulfonate groups obtained by chemical modification of chitosan useful in green synthesis of Au and Ag nanoparticles","authors":"M. Caldera-Villalobos, J. García-Serrano, A. A. Peláez-Cid, Ana M. Herrera-González","doi":"10.1002/app.45240","DOIUrl":null,"url":null,"abstract":"<div>\n \n <p>Usually the metal nanoparticles are obtained by different chemical reactions that are not environmentally friendly. This paper describes the synthesis of two polyelectrolytes with sulfonate groups in <i>ortho-</i>position and in <i>ortho-</i> and <i>para-</i>positions, which were obtained by chemical modification of chitosan. They were used in the green synthesis of Au and Ag nanoparticles by colloidal method in aqueous solution. Polyelectrolytes were used as reducing agents of Au<sup>3+</sup> and Ag<sup>+</sup> ions and as stabilizing agents of Ag and Au nanoparticles. The hydroxyl and imine groups in the polyelectrolytes are reducing agents of Au<sup>3+</sup> and Ag<sup>+</sup> ions while the sulfonate groups and the polymer backbone stabilized Au and Ag nanoparticles. Polyelectrolyte 1, which has sulfonate group in <i>ortho-</i>position, favors the obtaining of anisotropic Au nanoparticles with an average size of 19 nm. While the polyelectrolyte 2, with two sulfonate groups in the <i>ortho</i>- and <i>para-</i>positions, yielded quasi-spherical Au nanoparticles with an average size of 14 nm. In general, Ag nanoparticles stabilized with both polyelectrolytes, show quasi-spherical forms with good control in size. Finally, both polyelectrolytes have the ability to protect the Au and Ag nanoparticles allowing obtaining colloidal solutions that are stable for several months. © 2017 Wiley Periodicals, Inc. J. Appl. Polym. Sci. <b>2017</b>, <i>134</i>, 45240.</p>\n </div>","PeriodicalId":183,"journal":{"name":"Journal of Applied Polymer Science","volume":"134 38","pages":""},"PeriodicalIF":2.8000,"publicationDate":"2017-05-25","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/app.45240","citationCount":"9","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Journal of Applied Polymer Science","FirstCategoryId":"92","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/app.45240","RegionNum":3,"RegionCategory":"化学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"POLYMER SCIENCE","Score":null,"Total":0}
引用次数: 9
引用
批量引用
Abstract
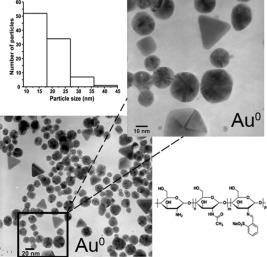
Usually the metal nanoparticles are obtained by different chemical reactions that are not environmentally friendly. This paper describes the synthesis of two polyelectrolytes with sulfonate groups in ortho- position and in ortho- and para- positions, which were obtained by chemical modification of chitosan. They were used in the green synthesis of Au and Ag nanoparticles by colloidal method in aqueous solution. Polyelectrolytes were used as reducing agents of Au3+ and Ag+ ions and as stabilizing agents of Ag and Au nanoparticles. The hydroxyl and imine groups in the polyelectrolytes are reducing agents of Au3+ and Ag+ ions while the sulfonate groups and the polymer backbone stabilized Au and Ag nanoparticles. Polyelectrolyte 1, which has sulfonate group in ortho- position, favors the obtaining of anisotropic Au nanoparticles with an average size of 19 nm. While the polyelectrolyte 2, with two sulfonate groups in the ortho - and para- positions, yielded quasi-spherical Au nanoparticles with an average size of 14 nm. In general, Ag nanoparticles stabilized with both polyelectrolytes, show quasi-spherical forms with good control in size. Finally, both polyelectrolytes have the ability to protect the Au and Ag nanoparticles allowing obtaining colloidal solutions that are stable for several months. © 2017 Wiley Periodicals, Inc. J. Appl. Polym. Sci. 2017 , 134 , 45240.
壳聚糖化学改性得到的磺酸基聚电解质可用于绿色合成金、银纳米粒子
通常,金属纳米颗粒是通过不同的化学反应获得的,这些化学反应对环境不友好。本文介绍了壳聚糖化学改性制备的两种邻位、邻位和对位磺酸基聚电解质。在水溶液中采用胶体法制备了金、银纳米粒子的绿色合成。用聚电解质作为Au3+和Ag+离子的还原剂和Ag和Au纳米粒子的稳定剂。聚电解质中的羟基和亚胺基是Au3+和Ag+离子的还原剂,而磺酸基和聚合物主链是Au和Ag纳米粒子的稳定剂。聚电解质1具有邻位磺酸基,有利于获得平均尺寸为19 nm的各向异性金纳米粒子。而聚电解质2,在邻位和对位上有两个磺酸基,产生的准球形金纳米粒子平均尺寸为14纳米。总的来说,两种聚电解质稳定的银纳米颗粒呈准球形,尺寸控制良好。最后,这两种聚电解质都有能力保护Au和Ag纳米颗粒,从而获得稳定数月的胶体溶液。©2017 Wiley期刊公司j:。变异较大。自然科学学报,2017,34(4):45240。
本文章由计算机程序翻译,如有差异,请以英文原文为准。