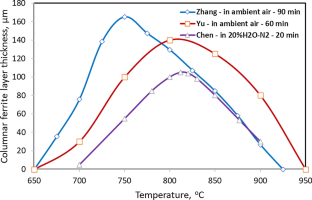
Recently there was a new wave of research activities studying the decarburization behavior of spring steels with the main focus on the formation mechanism of a columnar ferrite layer within a certain temperature range which could not be explained by conventional decarburization theories. A new theory successfully developed recently in interpreting the oxide scale reduction mechanism on steel was then developed further and applied to interpret the observed columnar ferrite formation on spring steels. The essence of the new theory is that steel decarburization in the presence of a FeO scale on the steel surface is caused and governed by the reaction between the FeO scale and dissolved carbon in the steel, and therefore, the carbon concentration on the steel surface is determined by the FeO-steel interface equilibrium and cannot be treated as negligible within the temperature range where ferrite is able to form, because the equilibrium interface carbon concentration is in the same magnitude as the carbon solubility in ferrite. The new theory and available solutions for different decarburization scenarios using decarburization of 60Si2MnA as an example are summarized in this review. Explanations are given to interpret discrepancies between experimental observations and theoretical predictions. New areas for future research are also identified.