Roxadustat is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor approved in several regions for the treatment of anemia of chronic kidney disease (CKD). ASPEN evaluated the efficacy, safety, and feasibility of roxadustat in patients with anemia of CKD in US dialysis organizations.
This open-label, single-arm study (NCT04484857) comprised a 6-week screening period, followed by 24 weeks of treatment (with optional extension ≤1 year) and a 4-week follow-up. Patients aged ≥18 years, receiving chronic dialysis, with hemoglobin (Hb) 9.0–12.0 g/dL if converting from erythropoiesis-stimulating agents (ESAs), or <10.0 g/dL if receiving ESAs for <6 weeks, received oral roxadustat three times weekly in-center. Primary efficacy endpoints included proportion of patients with mean Hb ≥10 g/dL, averaged over weeks 16–24, and mean Hb change from baseline to the average over weeks 16–24. Safety was also assessed.
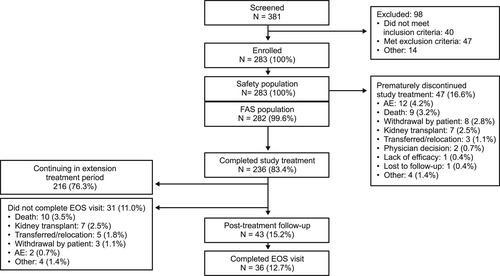
Overall, 283 patients were enrolled and treated, 282 (99.6%) were included in the full analysis set, and 216 (76.3%) continued into the extension period. Most patients enrolled were from DaVita sites (71%), with the rest from US Renal Care sites (29%). Mean (standard deviation [SD]) baseline Hb was 10.6 (0.7) g/dL. Nearly all patients were prior ESA users (n = 274; 97.2%). The proportion of patients with mean Hb ≥10 g/dL during weeks 16–24 was 83.7% (95% confidence interval 78.9–88.6). Mean (SD) Hb increase from baseline to the average over weeks 16–24 was 0.2 (1.0) g/dL. During the treatment period, 82 (29.0%) patients reported treatment-emergent serious adverse events (TESAEs). The most common TESAEs were COVID-19 pneumonia (n = 10; 3.5%), acute respiratory failure (n = 9; 3.2%), COVID-19 (n = 7; 2.5%), acute myocardial infarction (n = 7; 2.5%), and fluid overload (n = 6, 2.1%).
Roxadustat was effective in maintaining Hb in patients with anemia of CKD on dialysis in large, community-based dialysis organizations.