下载PDF
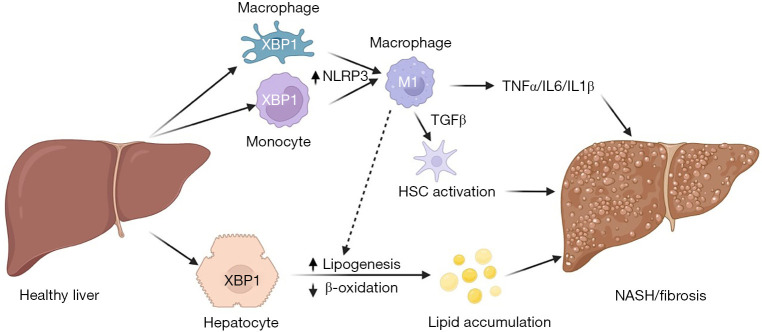
{"title":"Integrating stress signals-XBP1 as a novel mediator of intercellular crosstalk in non-alcoholic steatohepatitis.","authors":"Junjie Yu, Utpal B Pajvani","doi":"10.21037/tgh-22-73","DOIUrl":null,"url":null,"abstract":"© Translational Gastroenterology and Hepatology. All rights reserved. Transl Gastroenterol Hepatol 2023;8:13 | https://dx.doi.org/10.21037/tgh-22-73 Non-alcoholic steatohepatitis (NASH), the most advanced form of non-alcoholic fatty liver disease (NAFLD), is the most common chronic liver disease and will soon be the most common reason for liver transplantation as NASH has no approved pharmacotherapy. This unmet need is due in part to an incomplete understanding of mechanistic determinants of various aspects of NASH pathogenesis, and uncertain progression between a prevalent pre-NASH state (lipid accumulation) to the hepatocyte injury, inflammation and fibrosis that defines NASH (1). NASH develops in the context of obesity, but people of similar body weight and adiposity do not have identical risk of NASH development. This discordance highlights a genetic component to the disease, well-established for hepatic lipid accumulation from genome-wide association studies (GWAS), but less so for liver inflammation and fibrosis. One possibility is that heterogeneity in NASH arises from altered transcriptional regulation that may not be easily uncovered by GWAS, as multiple metabolic and developmental signaling pathways are preferentially dysregulated in NASH but not pre-NASH states (2). These and other studies have suggested that two or more “hits” are necessary for NASH development (3,4). In this conceptual framework, lipid accumulation in hepatocytes, termed steatosis, may (but not necessarily) prompt a second hit, for example, oxidative stress, which will trigger hepatocyte death, immune cell activation, and eventually, the deposition of collagen fibrils by activated hepatic stellate cells (HSCs). Other work suggests that NASH development is augmented by extrahepatic mechanisms, including gutand adipose tissue-derived proinflammatory factors, which may be synergetic. In fact, inflammation may theoretically precede or even provoke steatosis. Chronic endoplasmic reticulum (ER) stress is linked to pathogenesis of multiple diseases (5-7), including obesity-induced metabolic diseases such as type 2 diabetes (T2D) and NASH, suggesting a possible pathogenic role. ER stress is often associated with the accumulation of unfolded proteins in the ER, which bind to the binding immunoglobulin protein (BIP), and triggers three, parallel signaling pathways—inositol-requiring enzyme 1α (IRE1α), which activates c-Jun NH2-terminal kinase (JNK), causing apoptosis, and induces splicing of X-box binding protein 1 (XBP1), leading to transcriptional upregulation of genes involved in lipogenesis, folding and ER-associated degradation (ERAD); PKR-like ER kinase (PERK)-induced phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), which leads to increased activating transcription factor (ATF4), and upregulated gene expression associated with amino acid metabolism, anti-oxidative stress and apoptosis; and activating transcription factor 6α (ATF6α), which is further processed to release a transcriptionally active cytoplasmic domain, leading to increase of ERAD and protein folding associated gene expression (5). Collectively, these signaling pathways are thought to alleviate ER stress through multiple mechanisms, including degradation of unfolded proteins, inhibition of new translation, and increase in ER capacity, which collectively augment likelihood of cellular survival. In chronic, unresolved ER stress, apoptosis and cell death ensue (5,8). Editorial Commentary","PeriodicalId":23267,"journal":{"name":"Translational gastroenterology and hepatology","volume":"8 ","pages":"13"},"PeriodicalIF":3.0000,"publicationDate":"2023-01-01","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://ftp.ncbi.nlm.nih.gov/pub/pmc/oa_pdf/f7/3e/tgh-08-22-73.PMC10184033.pdf","citationCount":"1","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Translational gastroenterology and hepatology","FirstCategoryId":"3","ListUrlMain":"https://doi.org/10.21037/tgh-22-73","RegionNum":4,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"Medicine","Score":null,"Total":0}
引用次数: 1
引用
批量引用
Abstract
© Translational Gastroenterology and Hepatology. All rights reserved. Transl Gastroenterol Hepatol 2023;8:13 | https://dx.doi.org/10.21037/tgh-22-73 Non-alcoholic steatohepatitis (NASH), the most advanced form of non-alcoholic fatty liver disease (NAFLD), is the most common chronic liver disease and will soon be the most common reason for liver transplantation as NASH has no approved pharmacotherapy. This unmet need is due in part to an incomplete understanding of mechanistic determinants of various aspects of NASH pathogenesis, and uncertain progression between a prevalent pre-NASH state (lipid accumulation) to the hepatocyte injury, inflammation and fibrosis that defines NASH (1). NASH develops in the context of obesity, but people of similar body weight and adiposity do not have identical risk of NASH development. This discordance highlights a genetic component to the disease, well-established for hepatic lipid accumulation from genome-wide association studies (GWAS), but less so for liver inflammation and fibrosis. One possibility is that heterogeneity in NASH arises from altered transcriptional regulation that may not be easily uncovered by GWAS, as multiple metabolic and developmental signaling pathways are preferentially dysregulated in NASH but not pre-NASH states (2). These and other studies have suggested that two or more “hits” are necessary for NASH development (3,4). In this conceptual framework, lipid accumulation in hepatocytes, termed steatosis, may (but not necessarily) prompt a second hit, for example, oxidative stress, which will trigger hepatocyte death, immune cell activation, and eventually, the deposition of collagen fibrils by activated hepatic stellate cells (HSCs). Other work suggests that NASH development is augmented by extrahepatic mechanisms, including gutand adipose tissue-derived proinflammatory factors, which may be synergetic. In fact, inflammation may theoretically precede or even provoke steatosis. Chronic endoplasmic reticulum (ER) stress is linked to pathogenesis of multiple diseases (5-7), including obesity-induced metabolic diseases such as type 2 diabetes (T2D) and NASH, suggesting a possible pathogenic role. ER stress is often associated with the accumulation of unfolded proteins in the ER, which bind to the binding immunoglobulin protein (BIP), and triggers three, parallel signaling pathways—inositol-requiring enzyme 1α (IRE1α), which activates c-Jun NH2-terminal kinase (JNK), causing apoptosis, and induces splicing of X-box binding protein 1 (XBP1), leading to transcriptional upregulation of genes involved in lipogenesis, folding and ER-associated degradation (ERAD); PKR-like ER kinase (PERK)-induced phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), which leads to increased activating transcription factor (ATF4), and upregulated gene expression associated with amino acid metabolism, anti-oxidative stress and apoptosis; and activating transcription factor 6α (ATF6α), which is further processed to release a transcriptionally active cytoplasmic domain, leading to increase of ERAD and protein folding associated gene expression (5). Collectively, these signaling pathways are thought to alleviate ER stress through multiple mechanisms, including degradation of unfolded proteins, inhibition of new translation, and increase in ER capacity, which collectively augment likelihood of cellular survival. In chronic, unresolved ER stress, apoptosis and cell death ensue (5,8). Editorial Commentary