During treatment for acute lymphoblastic leukaemia (ALL), children are prone to musculoskeletal deterioration. However, non-invasive tools to measure muscle mass and intramuscular alterations are limited. In this study we explored the feasibility of muscle ultrasound in children with ALL. Additionally, we analysed whether automated ultrasound outcomes of muscle size and intramuscular fat infiltration (IMAT) were associated with appendicular skeletal muscle mass (ASMM), muscle strength and physical performance.
Children with ALL, aged 3–18 years were included during maintenance therapy. Bilateral images of the rectus femoris muscle were captured using a portable linear array transducer connected to a tablet. Subsequently, an automated image annotation software (MuscleSound) was used to estimate cross-sectional area, muscle thickness and IMAT. Feasibility was assessed using acceptance (percentage of children approached who were enrolled), practicality (percentage of children that completed the ultrasound measurement after enrolment) and implementation (percentage of children that had sufficient imaging to be processed and analysed by the software). Assessments of ASMM by bioimpedance analysis, muscle strength using handheld dynamometry and timed physical performance tests were administered at the same visit. Multivariable linear models were estimated to study the associations between muscle ultrasound outcomes and ASMM, strength and physical performance, adjusted for sex, age, body mass index and ALL treatment week.
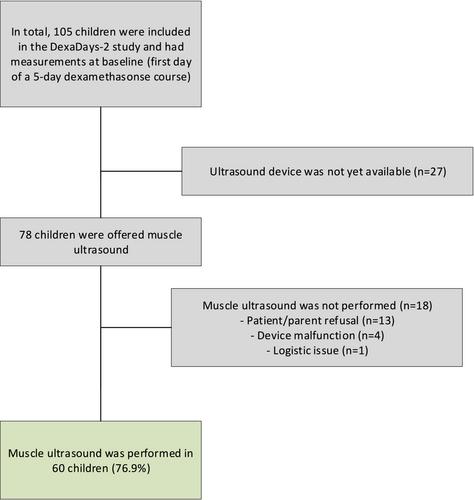
Muscle ultrasound was performed in 60 out of 73 invited patients (76.9%), of which 37 were boys (61.7%), and median age was 6.1 years (range: 3–18.8 years). The acceptance was 98.7%, practicality 77.9% and implementation was 100%. Patients who refused the examination (n = 13) were younger (median: 3.6, range: 3–11.2 years) compared with the 60 examined children (P = 0.0009). In multivariable models, cross-sectional area was associated with ASMM (β = 0.49 Z-score, 95% confidence interval [CI]:0.3,2.4), knee-extension strength (β = 16.9 Newton [N], 95% CI: 4.8, 28.9), walking performance (β = −0.46 s, 95% CI: −0.75, −0.18) and rising from the floor (β = −1.07 s, 95% CI: −1.71, −0.42). Muscle thickness was associated with ASMM (β = 0.14 Z-score, 95% CI: 0.04, 0.24), knee-extension strength (β = 4.73 N, 95% CI: 0.99, 8.47), walking performance (β = −0.13 s, 95% CI: −0.22, −0.04) and rising from the floor (β = −0.28 s, 95% CI: −0.48, −0.08). IMAT was associated with knee-extension strength (β = −6.84 N, 95% CI: −12.26, −1.41), walking performance (β = 0.2 s, 95% CI: 0.08, 0.32) and rising from the floor (β = 0.54 s, 95% CI: 0.27, 0.8). None of the muscle ultrasound outcomes was associated with handgrip strength.
Portable muscle ultrasound appears a feasible and useful tool to measure muscle size and intramuscular alterations in children with ALL. Validation studies using magnetic resonance imaging (gold standard) are necessary to confirm accuracy in paediatric populations.