Myostatin, encoded by the MSTN gene comprising 3 exons, is a potent negative regulator of skeletal muscle growth. Although a variety of myostatin inhibitors have been invented for increasing muscle mass in muscle wasting diseases, no effective inhibitor is currently available for clinical use. Myostatin isoforms in several animals have been reported to inhibit myostatin, but an isoform has never been identified for the human MSTN gene, a conserved gene among animals. Here, a splice variant of the human MSTN gene was explored.
Transcripts and proteins were analysed by reverse transcription-PCR amplification and western blotting, respectively. Proteins were expressed from expression plasmid. Myostatin signalling was assayed by the SMAD-responsive luciferase activity. Cell proliferation was assayed by the Cell Counting Kit-8 (CCK-8) assay and cell counting. Cell cycle was analysed by the FastFUCCI system.
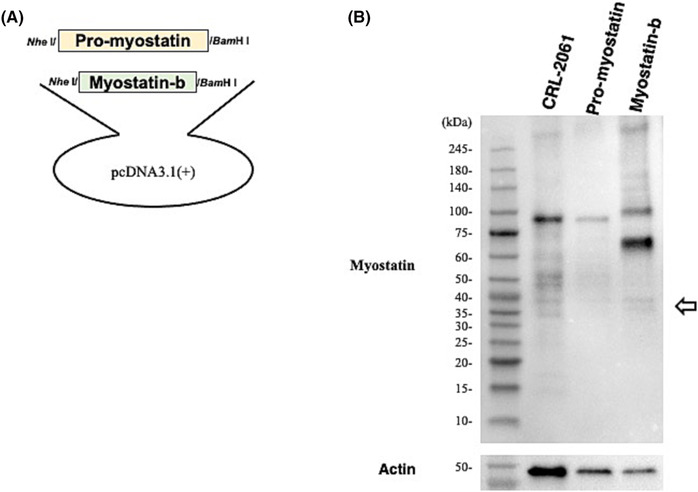
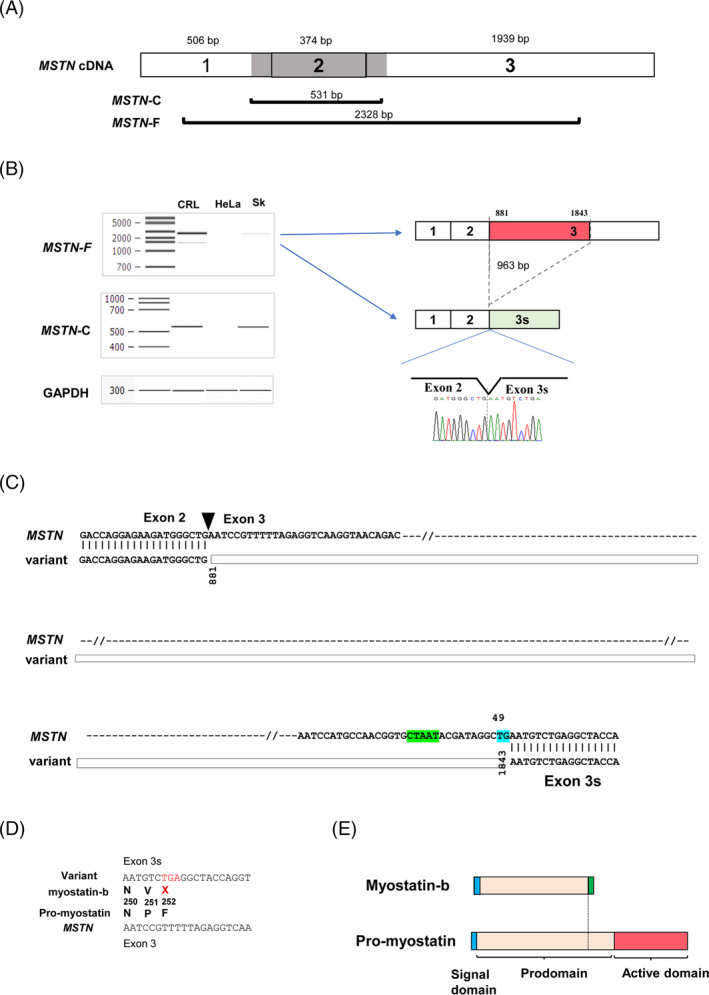
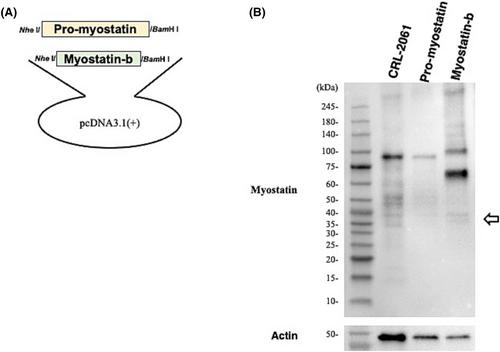
Reverse transcription-PCR amplification of the full-length MSTN transcript in CRL-2061 rhabdomyosarcoma cells revealed two bands consisting of a thick expected-size product and a thin additional small-size product. Sequencing of the small-size product showed a 963-bp deletion in the 5′ end of exon 3, creating exon 3s, which contained unusual splice acceptor TG dinucleotides. The novel variant was identified in other human cell lines, although it was not identified in skeletal muscle. The 251-amino acid isoform encoded by the novel variant (myostatin-b) was identified in CRL-2061 rhabdomyosarcoma cells. Transfection of a myostatin-b expression plasmid into CRL-2061 and myoblast cells inhibited endogenous myostatin signalling (44%, P < 0.001 and 63%, P < 0.001, respectively). Furthermore, myostatin-b inhibited myostatin signalling induced by recombinant myostatin (68.8%, P < 0.001). In remarkable contrast, myostatin-b did not inhibit the myostatin signalling induced by recombinant growth differentiation factor 11 (9.2%, P = 0.70), transforming growth factor β (+3.1%, P = 0.83) or activin A (+1.1%, P = 0.96). These results indicate the myostatin-specific inhibitory effect of myostatin-b. Notably, the expression of myostatin-b in myoblasts significantly enhanced cell proliferation higher than the mock-transfected cells by the CCK-8 and direct cell counting assays (60%, P < 0.05 and 39%, P < 0.05, respectively). Myostatin-b increased the percentage of S-phase cells significantly higher than that of the mock-transfected cells (53% vs. 80%, P < 0.05).
We cloned a novel human MSTN variant produced by unorthodox splicing. The variant encoded a novel myostatin isoform, myostatin-b, that inhibited myostatin signalling by myostatin-specific manner and enhanced myoblast proliferation by shifting cell cycle. Myostatin-b, which has myostatin-specific inhibitory activity, could be developed as a natural myostatin inhibitor.