求助PDF
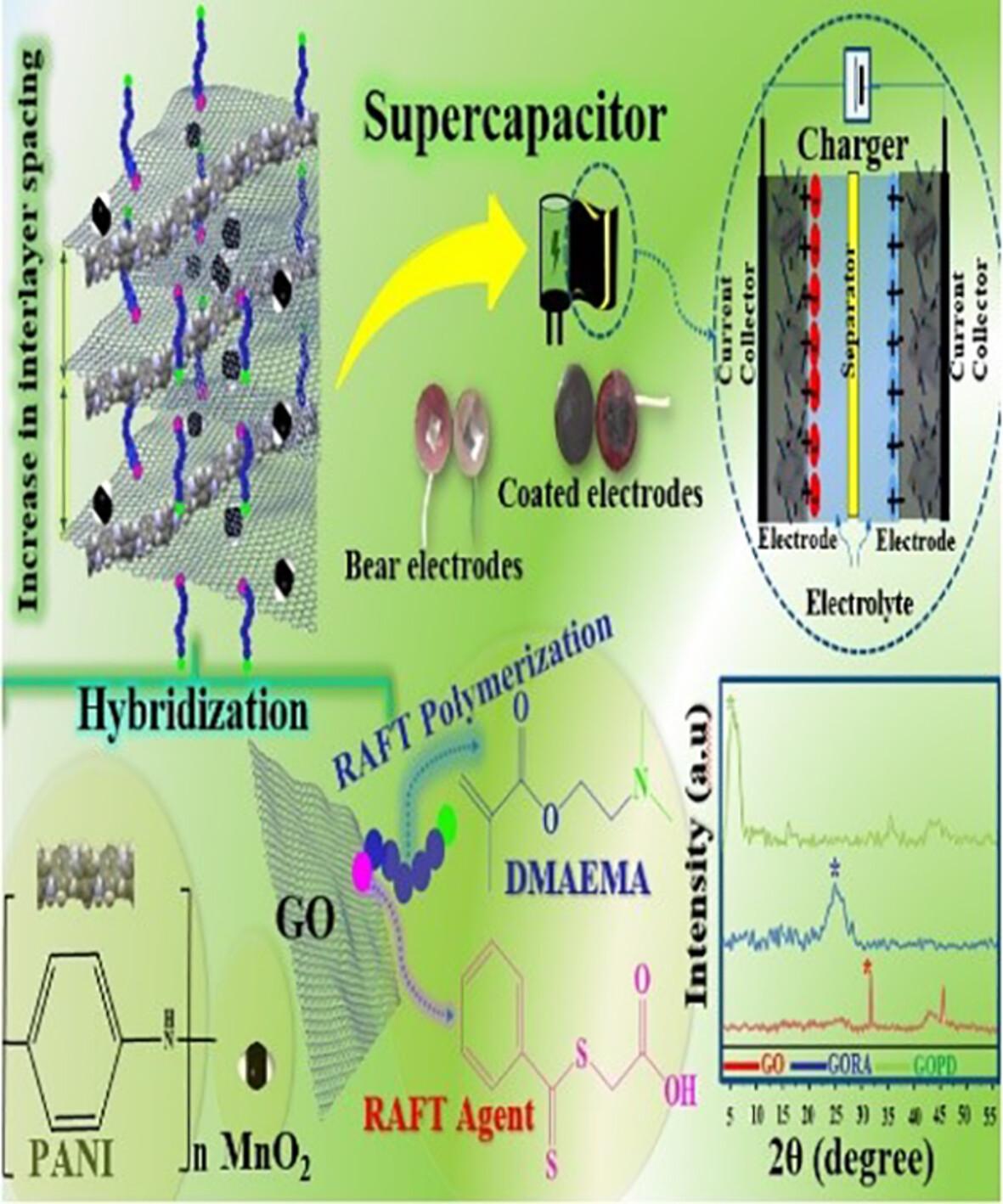
{"title":"作为超级电容器活性电极材料的 RAFT 聚合 DMAEMA 功能化氧化石墨烯、二氧化锰和聚苯胺三元纳米复合材料","authors":"Majid Moussaei, Vahid Haddadi-Asl, Hanie Ahmadi","doi":"10.1002/pi.6631","DOIUrl":null,"url":null,"abstract":"<p>Graphene and its derivatives are promising energy storage devices due to their high specific surface area, chemical and thermal durability and high charge transfer power. Still, their stacking and aggregate behavior can limit their practical application. To address this issue, reversible addition–fragmentation chain-transfer (RAFT) polymerization as controlled radical polymerization was applied to functionalize the surface of graphene oxide with poly(<i>N</i>,<i>N</i>-dimethylaminoethyl methacrylate) (PDMAEMA) via the ‘grafting from’ method. This strategy enhances the distance between graphene sheets by occupying physical volume while improving effective charge transfer through tertiary amine groups participating in the doping process. X-ray diffraction was used to determine interlayer spacing after polymer grafting, which increased from 0.28 to 1.71 nm after polymer grafting. The hybridization of materials with diverse properties was used to enhance charge transfer capability for supercapacitor applications. PDMAEMA-functionalized graphene oxide, nano-manganese dioxide and polyaniline were combined to create a successful nanocomposite as electrode active material. The morphological structure and chemical composition of the synthesized nanocomposite were analyzed, and its electrochemical performance was evaluated using cyclic voltammetry, galvanostatic charge–discharge and electrochemical impedance spectroscopy. The nanocomposite exhibited a maximum specific capacitance, energy density and power density of 364.72 F g<sup>−1</sup> (at a scan rate of 50 mV s<sup>−1</sup>), 239.08 Wh kg<sup>−1</sup> and 678.34 W kg<sup>−1</sup>, respectively. The final nanocomposite's energy storage capacity significantly increased compared to the individual components due to hybridization's synergistic impact, reducing charge transfer resistance. © 2024 Society of Industrial Chemistry.</p>","PeriodicalId":20404,"journal":{"name":"Polymer International","volume":"73 8","pages":"612-624"},"PeriodicalIF":2.9000,"publicationDate":"2024-03-13","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"0","resultStr":"{\"title\":\"Ternary nanocomposite of RAFT-polymerized DMAEMA-functionalized graphene oxide, manganese dioxide and polyaniline as active electrode material for supercapacitor\",\"authors\":\"Majid Moussaei, Vahid Haddadi-Asl, Hanie Ahmadi\",\"doi\":\"10.1002/pi.6631\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Graphene and its derivatives are promising energy storage devices due to their high specific surface area, chemical and thermal durability and high charge transfer power. Still, their stacking and aggregate behavior can limit their practical application. To address this issue, reversible addition–fragmentation chain-transfer (RAFT) polymerization as controlled radical polymerization was applied to functionalize the surface of graphene oxide with poly(<i>N</i>,<i>N</i>-dimethylaminoethyl methacrylate) (PDMAEMA) via the ‘grafting from’ method. This strategy enhances the distance between graphene sheets by occupying physical volume while improving effective charge transfer through tertiary amine groups participating in the doping process. X-ray diffraction was used to determine interlayer spacing after polymer grafting, which increased from 0.28 to 1.71 nm after polymer grafting. The hybridization of materials with diverse properties was used to enhance charge transfer capability for supercapacitor applications. PDMAEMA-functionalized graphene oxide, nano-manganese dioxide and polyaniline were combined to create a successful nanocomposite as electrode active material. The morphological structure and chemical composition of the synthesized nanocomposite were analyzed, and its electrochemical performance was evaluated using cyclic voltammetry, galvanostatic charge–discharge and electrochemical impedance spectroscopy. The nanocomposite exhibited a maximum specific capacitance, energy density and power density of 364.72 F g<sup>−1</sup> (at a scan rate of 50 mV s<sup>−1</sup>), 239.08 Wh kg<sup>−1</sup> and 678.34 W kg<sup>−1</sup>, respectively. The final nanocomposite's energy storage capacity significantly increased compared to the individual components due to hybridization's synergistic impact, reducing charge transfer resistance. © 2024 Society of Industrial Chemistry.</p>\",\"PeriodicalId\":20404,\"journal\":{\"name\":\"Polymer International\",\"volume\":\"73 8\",\"pages\":\"612-624\"},\"PeriodicalIF\":2.9000,\"publicationDate\":\"2024-03-13\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Polymer International\",\"FirstCategoryId\":\"92\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/pi.6631\",\"RegionNum\":4,\"RegionCategory\":\"化学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q2\",\"JCRName\":\"POLYMER SCIENCE\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Polymer International","FirstCategoryId":"92","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/pi.6631","RegionNum":4,"RegionCategory":"化学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"POLYMER SCIENCE","Score":null,"Total":0}
引用次数: 0
引用
批量引用