求助PDF
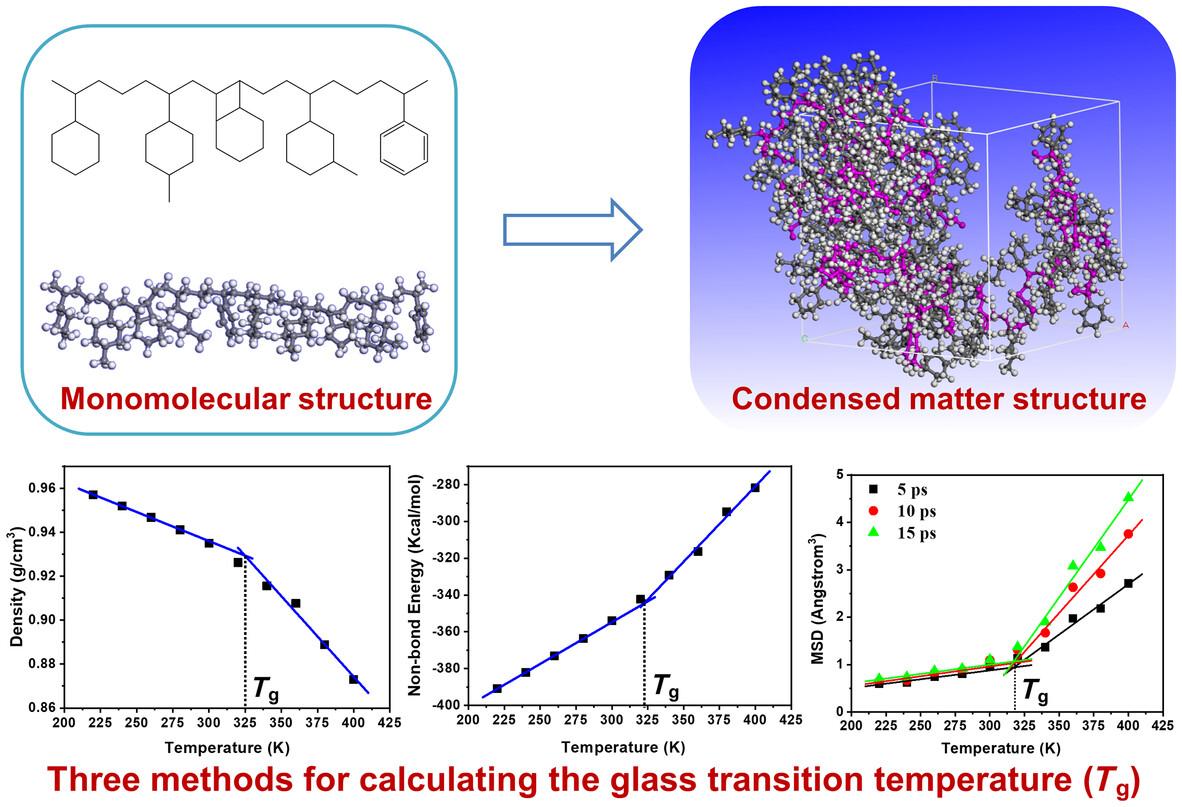
{"title":"通过全原子分子动力学模拟预测橡胶/二氧化硅和橡胶/树脂之间的玻璃化转变温度和溶解度参数","authors":"Qionghai Chen, Ziyi Zhang, Wanhui Huang, JiaJun Qu, Qi Zhang, Xiaohui Wu, Liqun Zhang, Jun Liu","doi":"10.1002/pi.6647","DOIUrl":null,"url":null,"abstract":"<p>Resin is a widely used additive in rubber composites, which not only improves the processing properties of the composites but also enhances their mechanical properties, rolling resistance and wear resistance. However, there are specific differences in compatibility among resin, rubber and silica, which directly affect the performance of the composite materials. In this work, we first computed the glass transition temperature (<span></span><math>\n <mrow>\n <msub>\n <mi>T</mi>\n <mi>g</mi>\n </msub>\n </mrow></math>) of five resins in styrene−butadiene rubber (SBR) composites to prove the reliability of the computational method. Then, we explored the effects of different components and resin types on <span></span><math>\n <mrow>\n <msub>\n <mi>T</mi>\n <mi>g</mi>\n </msub>\n </mrow></math> of SBR and found that the addition of silica can increase <span></span><math>\n <mrow>\n <msub>\n <mi>T</mi>\n <mi>g</mi>\n </msub>\n </mrow></math> due to weak attractive interactions between silica and rubber molecular chains, which restrict the movement of the molecular chains. Furthermore, using solubility parameters, we analyzed the compatibility of rubber and five different resins and found that all five resins had good compatibility with rubber, especially C5/C9 copolymerized petroleum resin and hydrogenated resin. Finally, we revealed that there is a mutually attractive force between resin and silica. In summary, understanding the interactions among resins, silica and rubber is crucial for optimizing the performance of composite materials. © 2024 Society of Chemical Industry.</p>","PeriodicalId":20404,"journal":{"name":"Polymer International","volume":"73 9","pages":"770-778"},"PeriodicalIF":2.9000,"publicationDate":"2024-05-10","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"0","resultStr":"{\"title\":\"Predicting the glass transition temperature and solubility parameter between rubber/silica and rubber/resins via all-atom molecular dynamics simulation\",\"authors\":\"Qionghai Chen, Ziyi Zhang, Wanhui Huang, JiaJun Qu, Qi Zhang, Xiaohui Wu, Liqun Zhang, Jun Liu\",\"doi\":\"10.1002/pi.6647\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Resin is a widely used additive in rubber composites, which not only improves the processing properties of the composites but also enhances their mechanical properties, rolling resistance and wear resistance. However, there are specific differences in compatibility among resin, rubber and silica, which directly affect the performance of the composite materials. In this work, we first computed the glass transition temperature (<span></span><math>\\n <mrow>\\n <msub>\\n <mi>T</mi>\\n <mi>g</mi>\\n </msub>\\n </mrow></math>) of five resins in styrene−butadiene rubber (SBR) composites to prove the reliability of the computational method. Then, we explored the effects of different components and resin types on <span></span><math>\\n <mrow>\\n <msub>\\n <mi>T</mi>\\n <mi>g</mi>\\n </msub>\\n </mrow></math> of SBR and found that the addition of silica can increase <span></span><math>\\n <mrow>\\n <msub>\\n <mi>T</mi>\\n <mi>g</mi>\\n </msub>\\n </mrow></math> due to weak attractive interactions between silica and rubber molecular chains, which restrict the movement of the molecular chains. Furthermore, using solubility parameters, we analyzed the compatibility of rubber and five different resins and found that all five resins had good compatibility with rubber, especially C5/C9 copolymerized petroleum resin and hydrogenated resin. Finally, we revealed that there is a mutually attractive force between resin and silica. In summary, understanding the interactions among resins, silica and rubber is crucial for optimizing the performance of composite materials. © 2024 Society of Chemical Industry.</p>\",\"PeriodicalId\":20404,\"journal\":{\"name\":\"Polymer International\",\"volume\":\"73 9\",\"pages\":\"770-778\"},\"PeriodicalIF\":2.9000,\"publicationDate\":\"2024-05-10\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Polymer International\",\"FirstCategoryId\":\"92\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/pi.6647\",\"RegionNum\":4,\"RegionCategory\":\"化学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q2\",\"JCRName\":\"POLYMER SCIENCE\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Polymer International","FirstCategoryId":"92","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/pi.6647","RegionNum":4,"RegionCategory":"化学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"POLYMER SCIENCE","Score":null,"Total":0}
引用次数: 0
引用
批量引用