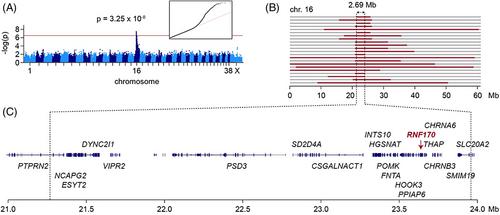
犬 RNF170 单碱基缺失自然形成的人类神经轴索营养不良症模型
Shawna R. Cook PhD, Cleo Schwarz MedVet, Julien Guevar DVM, MVM, DECVN, MRCVS, Charles-Antoine Assenmacher DVM, Msc, DACVP, Maeve Sheehy BS, Nathan Fanzone VMD, Molly E. Church MS, VMD, PhD, Leonardo Murgiano PhD, Margret L. Casal DVM, PhD, Vidhya Jagannathan PhD, Rodrigo Gutierrez-Quintana MVZ, MVM, Mark Lowrie MA, VetMB, MVM, DECVN, MRCVS, Frank Steffen DECVN, Tosso Leeb PhD, Kari J. Ekenstedt DVM, PhD
下载PDF
{"title":"犬 RNF170 单碱基缺失自然形成的人类神经轴索营养不良症模型","authors":"Shawna R. Cook PhD, Cleo Schwarz MedVet, Julien Guevar DVM, MVM, DECVN, MRCVS, Charles-Antoine Assenmacher DVM, Msc, DACVP, Maeve Sheehy BS, Nathan Fanzone VMD, Molly E. Church MS, VMD, PhD, Leonardo Murgiano PhD, Margret L. Casal DVM, PhD, Vidhya Jagannathan PhD, Rodrigo Gutierrez-Quintana MVZ, MVM, Mark Lowrie MA, VetMB, MVM, DECVN, MRCVS, Frank Steffen DECVN, Tosso Leeb PhD, Kari J. Ekenstedt DVM, PhD","doi":"10.1002/mds.29977","DOIUrl":null,"url":null,"abstract":"<div>\n \n \n <section>\n \n <h3> Background</h3>\n \n <p>Neuroaxonal dystrophy (NAD) is a group of inherited neurodegenerative disorders characterized primarily by the presence of spheroids (swollen axons) throughout the central nervous system. In humans, NAD is heterogeneous, both clinically and genetically. NAD has also been described to naturally occur in large animal models, such as dogs. A newly recognized disorder in Miniature American Shepherd dogs (MAS), consisting of a slowly progressive neurodegenerative syndrome, was diagnosed as NAD via histopathology.</p>\n </section>\n \n <section>\n \n <h3> Objectives</h3>\n \n <p>To describe the clinical and pathological phenotype together with the identification of the underlying genetic cause.</p>\n </section>\n \n <section>\n \n <h3> Methods</h3>\n \n <p>Clinical and postmortem evaluations, together with a genome-wide association study and autozygosity mapping approach, followed by whole-genome sequencing.</p>\n </section>\n \n <section>\n \n <h3> Results</h3>\n \n <p>Affected dogs were typically young adults and displayed an abnormal gait characterized by pelvic limb weakness and ataxia. The underlying genetic cause was identified as a 1-bp (base pair) deletion in <i>RNF170</i> encoding ring finger protein 170, which perfectly segregates in an autosomal recessive pattern. This deletion is predicted to create a frameshift (XM_038559916.1:c.367delG) and early truncation of the RNF170 protein (XP_038415844.1:(p.Ala123Glnfs*11)). The age of this canine <i>RNF170</i> variant was estimated at ~30 years, before the reproductive isolation of the MAS breed.</p>\n </section>\n \n <section>\n \n <h3> Conclusions</h3>\n \n <p><i>RNF170</i> variants were previously identified in human patients with autosomal recessive spastic paraplegia-85 (SPG85); this clinical phenotype shows similarities to the dogs described herein. We therefore propose that this novel MAS NAD could serve as an excellent large animal model for equivalent human diseases, particularly since affected dogs demonstrate a relatively long lifespan, which represents an opportunity for therapeutic trials. © 2024 The Author(s). <i>Movement Disorders</i> published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.</p>\n </section>\n </div>","PeriodicalId":213,"journal":{"name":"Movement Disorders","volume":"39 11","pages":"2049-2057"},"PeriodicalIF":7.4000,"publicationDate":"2024-08-23","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/mds.29977","citationCount":"0","resultStr":"{\"title\":\"Canine RNF170 Single Base Deletion in a Naturally Occurring Model for Human Neuroaxonal Dystrophy\",\"authors\":\"Shawna R. Cook PhD, Cleo Schwarz MedVet, Julien Guevar DVM, MVM, DECVN, MRCVS, Charles-Antoine Assenmacher DVM, Msc, DACVP, Maeve Sheehy BS, Nathan Fanzone VMD, Molly E. Church MS, VMD, PhD, Leonardo Murgiano PhD, Margret L. Casal DVM, PhD, Vidhya Jagannathan PhD, Rodrigo Gutierrez-Quintana MVZ, MVM, Mark Lowrie MA, VetMB, MVM, DECVN, MRCVS, Frank Steffen DECVN, Tosso Leeb PhD, Kari J. Ekenstedt DVM, PhD\",\"doi\":\"10.1002/mds.29977\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<div>\\n \\n \\n <section>\\n \\n <h3> Background</h3>\\n \\n <p>Neuroaxonal dystrophy (NAD) is a group of inherited neurodegenerative disorders characterized primarily by the presence of spheroids (swollen axons) throughout the central nervous system. In humans, NAD is heterogeneous, both clinically and genetically. NAD has also been described to naturally occur in large animal models, such as dogs. A newly recognized disorder in Miniature American Shepherd dogs (MAS), consisting of a slowly progressive neurodegenerative syndrome, was diagnosed as NAD via histopathology.</p>\\n </section>\\n \\n <section>\\n \\n <h3> Objectives</h3>\\n \\n <p>To describe the clinical and pathological phenotype together with the identification of the underlying genetic cause.</p>\\n </section>\\n \\n <section>\\n \\n <h3> Methods</h3>\\n \\n <p>Clinical and postmortem evaluations, together with a genome-wide association study and autozygosity mapping approach, followed by whole-genome sequencing.</p>\\n </section>\\n \\n <section>\\n \\n <h3> Results</h3>\\n \\n <p>Affected dogs were typically young adults and displayed an abnormal gait characterized by pelvic limb weakness and ataxia. The underlying genetic cause was identified as a 1-bp (base pair) deletion in <i>RNF170</i> encoding ring finger protein 170, which perfectly segregates in an autosomal recessive pattern. This deletion is predicted to create a frameshift (XM_038559916.1:c.367delG) and early truncation of the RNF170 protein (XP_038415844.1:(p.Ala123Glnfs*11)). The age of this canine <i>RNF170</i> variant was estimated at ~30 years, before the reproductive isolation of the MAS breed.</p>\\n </section>\\n \\n <section>\\n \\n <h3> Conclusions</h3>\\n \\n <p><i>RNF170</i> variants were previously identified in human patients with autosomal recessive spastic paraplegia-85 (SPG85); this clinical phenotype shows similarities to the dogs described herein. We therefore propose that this novel MAS NAD could serve as an excellent large animal model for equivalent human diseases, particularly since affected dogs demonstrate a relatively long lifespan, which represents an opportunity for therapeutic trials. © 2024 The Author(s). <i>Movement Disorders</i> published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.</p>\\n </section>\\n </div>\",\"PeriodicalId\":213,\"journal\":{\"name\":\"Movement Disorders\",\"volume\":\"39 11\",\"pages\":\"2049-2057\"},\"PeriodicalIF\":7.4000,\"publicationDate\":\"2024-08-23\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/mds.29977\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Movement Disorders\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/mds.29977\",\"RegionNum\":1,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"CLINICAL NEUROLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Movement Disorders","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/mds.29977","RegionNum":1,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"CLINICAL NEUROLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用