求助PDF
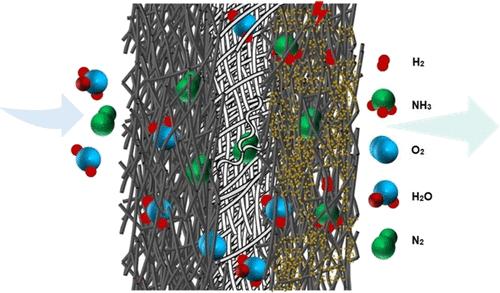
{"title":"通过超酸性微滴的传输提高电化学氮还原能力","authors":"Amir H. Aslambakhsh, Yuecheng Zhang, Sandra E. Kentish, Colin A. Scholes","doi":"10.1021/acsaem.4c01575","DOIUrl":null,"url":null,"abstract":"Electrocatalysts with a small overpotential hold a clear advantage in energy conversion efficiency. However, in electrochemical nitrogen reduction reactions (eNRRs), the primary challenge remains the issue of low selectivity. This study presents a gas-through cell assembly for eNRR to advance sustainable ammonia synthesis. The assembly introduces superacidic microdroplets via nitrogen gas, enhancing nitrogen concentration on the catalyst surface and amplifying nitrogen electrofixation rates. Catalyst deposition on a gas diffusion layer surface with a hydrophobic polymer binder enables microdroplet delivery to the working electrode surface through an ultrasonic nebulizer. Investigating parameters such as flow rate, water microdroplet content, temperature, pH, and applied potential provides valuable insights into eNRR performance. Unlike conventional H-cell setups, the proton concentration of the nebulizer flow emerges as the primary limiting factor in the gas-through cell assembly, impacting ammonia yield rate and Faradaic efficiency. Superacidic droplets enhance ammonia production, but further reducing pH increases hydrogen generation, lowering Faradaic efficiency toward ammonia. Higher temperatures accelerate ammonia production but reduce the Faradaic efficiency due to increased competition from the hydrogen evolution reaction, while elevated potentials initially boost eNRR but drop selectivity due to competing reactions. An optimum ammonia yield rate and Faradaic efficiency of 24.2 ± 2.4 <i></i><span style=\"color: inherit;\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\" display=\"inline\"><mi>&#x3BC;</mi><msub><mi mathvariant=\"normal\">g</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub><msup><msub><mi>mg</mi><mrow><mi>cata.</mi></mrow></msub><mrow><mo>&#x2212;</mo><mn>1</mn></mrow></msup><msup><mi mathvariant=\"normal\">h</mi><mrow><mo>&#x2212;</mo><mn>1</mn></mrow></msup></math>' role=\"presentation\" style=\"position: relative;\" tabindex=\"0\"><nobr aria-hidden=\"true\"><span style=\"width: 8.185em; display: inline-block;\"><span style=\"display: inline-block; position: relative; width: 7.446em; height: 0px; font-size: 110%;\"><span style=\"position: absolute; clip: rect(1.31em, 1007.45em, 2.787em, -999.997em); top: -2.327em; left: 0em;\"><span><span style=\"font-family: STIXMathJax_Normal-italic;\">𝜇</span><span><span style=\"display: inline-block; position: relative; width: 1.878em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1000.46em, 4.378em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-family: STIXMathJax_Main;\">g</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.804em; left: 0.514em;\"><span><span><span style=\"display: inline-block; position: relative; width: 1.31em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1001.03em, 4.151em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">NH</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.861em; left: 1.026em;\"><span style=\"font-size: 50%; font-family: STIXMathJax_Main;\">3</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span><span><span style=\"display: inline-block; position: relative; width: 3.582em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1002.67em, 4.435em, -999.997em); top: -3.974em; left: 0em;\"><span><span style=\"display: inline-block; position: relative; width: 2.673em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1001.25em, 4.378em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-family: STIXMathJax_Main;\">mg</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.747em; left: 1.253em;\"><span><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">cata.</span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -4.315em; left: 2.673em;\"><span><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">−</span><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">1</span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span><span><span style=\"display: inline-block; position: relative; width: 1.423em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.128em, 1000.51em, 4.151em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-family: STIXMathJax_Main;\">h</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -4.315em; left: 0.514em;\"><span><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">−</span><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">1</span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 2.332em;\"></span></span></span><span style=\"display: inline-block; overflow: hidden; vertical-align: -0.372em; border-left: 0px solid; width: 0px; height: 1.316em;\"></span></span></nobr><span role=\"presentation\"><math display=\"inline\" xmlns=\"http://www.w3.org/1998/Math/MathML\"><mi>μ</mi><msub><mi mathvariant=\"normal\">g</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub><msup><msub><mi>mg</mi><mrow><mi>cata.</mi></mrow></msub><mrow><mo>−</mo><mn>1</mn></mrow></msup><msup><mi mathvariant=\"normal\">h</mi><mrow><mo>−</mo><mn>1</mn></mrow></msup></math></span></span><script type=\"math/mml\"><math display=\"inline\"><mi>μ</mi><msub><mi mathvariant=\"normal\">g</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub><msup><msub><mi>mg</mi><mrow><mi>cata.</mi></mrow></msub><mrow><mo>−</mo><mn>1</mn></mrow></msup><msup><mi mathvariant=\"normal\">h</mi><mrow><mo>−</mo><mn>1</mn></mrow></msup></math></script> and 27 ± 4.4% were achieved, with 50 mL/min total flow rate and 50% volume microdroplet content, respectively; the ammonia synthesis rate reached as high as 37.6 ± 4 <i></i><span style=\"color: inherit;\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\" display=\"inline\"><mi>&#x3BC;</mi><msub><mi mathvariant=\"normal\">g</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub><msup><msub><mi>mg</mi><mrow><mi>cata.</mi></mrow></msub><mrow><mo>&#x2212;</mo><mn>1</mn></mrow></msup><msup><mi mathvariant=\"normal\">h</mi><mrow><mo>&#x2212;</mo><mn>1</mn></mrow></msup></math>' role=\"presentation\" style=\"position: relative;\" tabindex=\"0\"><nobr aria-hidden=\"true\"><span style=\"width: 8.185em; display: inline-block;\"><span style=\"display: inline-block; position: relative; width: 7.446em; height: 0px; font-size: 110%;\"><span style=\"position: absolute; clip: rect(1.31em, 1007.45em, 2.787em, -999.997em); top: -2.327em; left: 0em;\"><span><span style=\"font-family: STIXMathJax_Normal-italic;\">𝜇</span><span><span style=\"display: inline-block; position: relative; width: 1.878em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1000.46em, 4.378em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-family: STIXMathJax_Main;\">g</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.804em; left: 0.514em;\"><span><span><span style=\"display: inline-block; position: relative; width: 1.31em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1001.03em, 4.151em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">NH</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.861em; left: 1.026em;\"><span style=\"font-size: 50%; font-family: STIXMathJax_Main;\">3</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span><span><span style=\"display: inline-block; position: relative; width: 3.582em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1002.67em, 4.435em, -999.997em); top: -3.974em; left: 0em;\"><span><span style=\"display: inline-block; position: relative; width: 2.673em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1001.25em, 4.378em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-family: STIXMathJax_Main;\">mg</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.747em; left: 1.253em;\"><span><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">cata.</span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -4.315em; left: 2.673em;\"><span><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">−</span><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">1</span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span><span><span style=\"display: inline-block; position: relative; width: 1.423em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.128em, 1000.51em, 4.151em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-family: STIXMathJax_Main;\">h</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -4.315em; left: 0.514em;\"><span><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">−</span><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">1</span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 2.332em;\"></span></span></span><span style=\"display: inline-block; overflow: hidden; vertical-align: -0.372em; border-left: 0px solid; width: 0px; height: 1.316em;\"></span></span></nobr><span role=\"presentation\"><math display=\"inline\" xmlns=\"http://www.w3.org/1998/Math/MathML\"><mi>μ</mi><msub><mi mathvariant=\"normal\">g</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub><msup><msub><mi>mg</mi><mrow><mi>cata.</mi></mrow></msub><mrow><mo>−</mo><mn>1</mn></mrow></msup><msup><mi mathvariant=\"normal\">h</mi><mrow><mo>−</mo><mn>1</mn></mrow></msup></math></span></span><script type=\"math/mml\"><math display=\"inline\"><mi>μ</mi><msub><mi mathvariant=\"normal\">g</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub><msup><msub><mi>mg</mi><mrow><mi>cata.</mi></mrow></msub><mrow><mo>−</mo><mn>1</mn></mrow></msup><msup><mi mathvariant=\"normal\">h</mi><mrow><mo>−</mo><mn>1</mn></mrow></msup></math></script> at 85 °C, while the best Faradaic efficiency of 58.2 ± 9.3% was observed at pH = 2.8 ± 0.1 under −2 V applied potential and ambient pressure. This study enhances our understanding of gas-through electrochemical nitrogen fixation, providing invaluable insights for the development of efficient and sustainable ammonia synthesis strategies.","PeriodicalId":5,"journal":{"name":"ACS Applied Materials & Interfaces","volume":null,"pages":null},"PeriodicalIF":8.3000,"publicationDate":"2024-09-11","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"0","resultStr":"{\"title\":\"Enhanced Electrochemical Nitrogen Reduction via the Transport of Superacidic Microdroplets\",\"authors\":\"Amir H. Aslambakhsh, Yuecheng Zhang, Sandra E. Kentish, Colin A. Scholes\",\"doi\":\"10.1021/acsaem.4c01575\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"Electrocatalysts with a small overpotential hold a clear advantage in energy conversion efficiency. However, in electrochemical nitrogen reduction reactions (eNRRs), the primary challenge remains the issue of low selectivity. This study presents a gas-through cell assembly for eNRR to advance sustainable ammonia synthesis. The assembly introduces superacidic microdroplets via nitrogen gas, enhancing nitrogen concentration on the catalyst surface and amplifying nitrogen electrofixation rates. Catalyst deposition on a gas diffusion layer surface with a hydrophobic polymer binder enables microdroplet delivery to the working electrode surface through an ultrasonic nebulizer. Investigating parameters such as flow rate, water microdroplet content, temperature, pH, and applied potential provides valuable insights into eNRR performance. Unlike conventional H-cell setups, the proton concentration of the nebulizer flow emerges as the primary limiting factor in the gas-through cell assembly, impacting ammonia yield rate and Faradaic efficiency. Superacidic droplets enhance ammonia production, but further reducing pH increases hydrogen generation, lowering Faradaic efficiency toward ammonia. Higher temperatures accelerate ammonia production but reduce the Faradaic efficiency due to increased competition from the hydrogen evolution reaction, while elevated potentials initially boost eNRR but drop selectivity due to competing reactions. An optimum ammonia yield rate and Faradaic efficiency of 24.2 ± 2.4 <i></i><span style=\\\"color: inherit;\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\" display=\\\"inline\\\"><mi>&#x3BC;</mi><msub><mi mathvariant=\\\"normal\\\">g</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub><msup><msub><mi>mg</mi><mrow><mi>cata.</mi></mrow></msub><mrow><mo>&#x2212;</mo><mn>1</mn></mrow></msup><msup><mi mathvariant=\\\"normal\\\">h</mi><mrow><mo>&#x2212;</mo><mn>1</mn></mrow></msup></math>' role=\\\"presentation\\\" style=\\\"position: relative;\\\" tabindex=\\\"0\\\"><nobr aria-hidden=\\\"true\\\"><span style=\\\"width: 8.185em; display: inline-block;\\\"><span style=\\\"display: inline-block; position: relative; width: 7.446em; height: 0px; font-size: 110%;\\\"><span style=\\\"position: absolute; clip: rect(1.31em, 1007.45em, 2.787em, -999.997em); top: -2.327em; left: 0em;\\\"><span><span style=\\\"font-family: STIXMathJax_Normal-italic;\\\">𝜇</span><span><span style=\\\"display: inline-block; position: relative; width: 1.878em; height: 0px;\\\"><span style=\\\"position: absolute; clip: rect(3.355em, 1000.46em, 4.378em, -999.997em); top: -3.974em; left: 0em;\\\"><span style=\\\"font-family: STIXMathJax_Main;\\\">g</span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span><span style=\\\"position: absolute; top: -3.804em; left: 0.514em;\\\"><span><span><span style=\\\"display: inline-block; position: relative; width: 1.31em; height: 0px;\\\"><span style=\\\"position: absolute; clip: rect(3.355em, 1001.03em, 4.151em, -999.997em); top: -3.974em; left: 0em;\\\"><span style=\\\"font-size: 70.7%; font-family: STIXMathJax_Main;\\\">NH</span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span><span style=\\\"position: absolute; top: -3.861em; left: 1.026em;\\\"><span style=\\\"font-size: 50%; font-family: STIXMathJax_Main;\\\">3</span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span></span></span></span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span></span></span><span><span style=\\\"display: inline-block; position: relative; width: 3.582em; height: 0px;\\\"><span style=\\\"position: absolute; clip: rect(3.355em, 1002.67em, 4.435em, -999.997em); top: -3.974em; left: 0em;\\\"><span><span style=\\\"display: inline-block; position: relative; width: 2.673em; height: 0px;\\\"><span style=\\\"position: absolute; clip: rect(3.355em, 1001.25em, 4.378em, -999.997em); top: -3.974em; left: 0em;\\\"><span style=\\\"font-family: STIXMathJax_Main;\\\">mg</span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span><span style=\\\"position: absolute; top: -3.747em; left: 1.253em;\\\"><span><span style=\\\"font-size: 70.7%; font-family: STIXMathJax_Main;\\\">cata.</span></span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span></span></span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span><span style=\\\"position: absolute; top: -4.315em; left: 2.673em;\\\"><span><span style=\\\"font-size: 70.7%; font-family: STIXMathJax_Main;\\\">−</span><span style=\\\"font-size: 70.7%; font-family: STIXMathJax_Main;\\\">1</span></span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span></span></span><span><span style=\\\"display: inline-block; position: relative; width: 1.423em; height: 0px;\\\"><span style=\\\"position: absolute; clip: rect(3.128em, 1000.51em, 4.151em, -999.997em); top: -3.974em; left: 0em;\\\"><span style=\\\"font-family: STIXMathJax_Main;\\\">h</span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span><span style=\\\"position: absolute; top: -4.315em; left: 0.514em;\\\"><span><span style=\\\"font-size: 70.7%; font-family: STIXMathJax_Main;\\\">−</span><span style=\\\"font-size: 70.7%; font-family: STIXMathJax_Main;\\\">1</span></span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span></span></span></span><span style=\\\"display: inline-block; width: 0px; height: 2.332em;\\\"></span></span></span><span style=\\\"display: inline-block; overflow: hidden; vertical-align: -0.372em; border-left: 0px solid; width: 0px; height: 1.316em;\\\"></span></span></nobr><span role=\\\"presentation\\\"><math display=\\\"inline\\\" xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mi>μ</mi><msub><mi mathvariant=\\\"normal\\\">g</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub><msup><msub><mi>mg</mi><mrow><mi>cata.</mi></mrow></msub><mrow><mo>−</mo><mn>1</mn></mrow></msup><msup><mi mathvariant=\\\"normal\\\">h</mi><mrow><mo>−</mo><mn>1</mn></mrow></msup></math></span></span><script type=\\\"math/mml\\\"><math display=\\\"inline\\\"><mi>μ</mi><msub><mi mathvariant=\\\"normal\\\">g</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub><msup><msub><mi>mg</mi><mrow><mi>cata.</mi></mrow></msub><mrow><mo>−</mo><mn>1</mn></mrow></msup><msup><mi mathvariant=\\\"normal\\\">h</mi><mrow><mo>−</mo><mn>1</mn></mrow></msup></math></script> and 27 ± 4.4% were achieved, with 50 mL/min total flow rate and 50% volume microdroplet content, respectively; the ammonia synthesis rate reached as high as 37.6 ± 4 <i></i><span style=\\\"color: inherit;\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\" display=\\\"inline\\\"><mi>&#x3BC;</mi><msub><mi mathvariant=\\\"normal\\\">g</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub><msup><msub><mi>mg</mi><mrow><mi>cata.</mi></mrow></msub><mrow><mo>&#x2212;</mo><mn>1</mn></mrow></msup><msup><mi mathvariant=\\\"normal\\\">h</mi><mrow><mo>&#x2212;</mo><mn>1</mn></mrow></msup></math>' role=\\\"presentation\\\" style=\\\"position: relative;\\\" tabindex=\\\"0\\\"><nobr aria-hidden=\\\"true\\\"><span style=\\\"width: 8.185em; display: inline-block;\\\"><span style=\\\"display: inline-block; position: relative; width: 7.446em; height: 0px; font-size: 110%;\\\"><span style=\\\"position: absolute; clip: rect(1.31em, 1007.45em, 2.787em, -999.997em); top: -2.327em; left: 0em;\\\"><span><span style=\\\"font-family: STIXMathJax_Normal-italic;\\\">𝜇</span><span><span style=\\\"display: inline-block; position: relative; width: 1.878em; height: 0px;\\\"><span style=\\\"position: absolute; clip: rect(3.355em, 1000.46em, 4.378em, -999.997em); top: -3.974em; left: 0em;\\\"><span style=\\\"font-family: STIXMathJax_Main;\\\">g</span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span><span style=\\\"position: absolute; top: -3.804em; left: 0.514em;\\\"><span><span><span style=\\\"display: inline-block; position: relative; width: 1.31em; height: 0px;\\\"><span style=\\\"position: absolute; clip: rect(3.355em, 1001.03em, 4.151em, -999.997em); top: -3.974em; left: 0em;\\\"><span style=\\\"font-size: 70.7%; font-family: STIXMathJax_Main;\\\">NH</span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span><span style=\\\"position: absolute; top: -3.861em; left: 1.026em;\\\"><span style=\\\"font-size: 50%; font-family: STIXMathJax_Main;\\\">3</span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span></span></span></span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span></span></span><span><span style=\\\"display: inline-block; position: relative; width: 3.582em; height: 0px;\\\"><span style=\\\"position: absolute; clip: rect(3.355em, 1002.67em, 4.435em, -999.997em); top: -3.974em; left: 0em;\\\"><span><span style=\\\"display: inline-block; position: relative; width: 2.673em; height: 0px;\\\"><span style=\\\"position: absolute; clip: rect(3.355em, 1001.25em, 4.378em, -999.997em); top: -3.974em; left: 0em;\\\"><span style=\\\"font-family: STIXMathJax_Main;\\\">mg</span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span><span style=\\\"position: absolute; top: -3.747em; left: 1.253em;\\\"><span><span style=\\\"font-size: 70.7%; font-family: STIXMathJax_Main;\\\">cata.</span></span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span></span></span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span><span style=\\\"position: absolute; top: -4.315em; left: 2.673em;\\\"><span><span style=\\\"font-size: 70.7%; font-family: STIXMathJax_Main;\\\">−</span><span style=\\\"font-size: 70.7%; font-family: STIXMathJax_Main;\\\">1</span></span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span></span></span><span><span style=\\\"display: inline-block; position: relative; width: 1.423em; height: 0px;\\\"><span style=\\\"position: absolute; clip: rect(3.128em, 1000.51em, 4.151em, -999.997em); top: -3.974em; left: 0em;\\\"><span style=\\\"font-family: STIXMathJax_Main;\\\">h</span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span><span style=\\\"position: absolute; top: -4.315em; left: 0.514em;\\\"><span><span style=\\\"font-size: 70.7%; font-family: STIXMathJax_Main;\\\">−</span><span style=\\\"font-size: 70.7%; font-family: STIXMathJax_Main;\\\">1</span></span><span style=\\\"display: inline-block; width: 0px; height: 3.98em;\\\"></span></span></span></span></span><span style=\\\"display: inline-block; width: 0px; height: 2.332em;\\\"></span></span></span><span style=\\\"display: inline-block; overflow: hidden; vertical-align: -0.372em; border-left: 0px solid; width: 0px; height: 1.316em;\\\"></span></span></nobr><span role=\\\"presentation\\\"><math display=\\\"inline\\\" xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mi>μ</mi><msub><mi mathvariant=\\\"normal\\\">g</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub><msup><msub><mi>mg</mi><mrow><mi>cata.</mi></mrow></msub><mrow><mo>−</mo><mn>1</mn></mrow></msup><msup><mi mathvariant=\\\"normal\\\">h</mi><mrow><mo>−</mo><mn>1</mn></mrow></msup></math></span></span><script type=\\\"math/mml\\\"><math display=\\\"inline\\\"><mi>μ</mi><msub><mi mathvariant=\\\"normal\\\">g</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub><msup><msub><mi>mg</mi><mrow><mi>cata.</mi></mrow></msub><mrow><mo>−</mo><mn>1</mn></mrow></msup><msup><mi mathvariant=\\\"normal\\\">h</mi><mrow><mo>−</mo><mn>1</mn></mrow></msup></math></script> at 85 °C, while the best Faradaic efficiency of 58.2 ± 9.3% was observed at pH = 2.8 ± 0.1 under −2 V applied potential and ambient pressure. This study enhances our understanding of gas-through electrochemical nitrogen fixation, providing invaluable insights for the development of efficient and sustainable ammonia synthesis strategies.\",\"PeriodicalId\":5,\"journal\":{\"name\":\"ACS Applied Materials & Interfaces\",\"volume\":null,\"pages\":null},\"PeriodicalIF\":8.3000,\"publicationDate\":\"2024-09-11\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"ACS Applied Materials & Interfaces\",\"FirstCategoryId\":\"88\",\"ListUrlMain\":\"https://doi.org/10.1021/acsaem.4c01575\",\"RegionNum\":2,\"RegionCategory\":\"材料科学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"MATERIALS SCIENCE, MULTIDISCIPLINARY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"ACS Applied Materials & Interfaces","FirstCategoryId":"88","ListUrlMain":"https://doi.org/10.1021/acsaem.4c01575","RegionNum":2,"RegionCategory":"材料科学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"MATERIALS SCIENCE, MULTIDISCIPLINARY","Score":null,"Total":0}
引用次数: 0
引用
批量引用