求助PDF
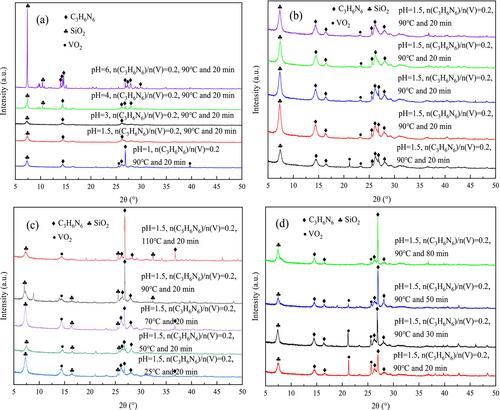
{"title":"以三聚氰胺为沉淀剂从溶液中高效快速沉淀钒","authors":"Bo Wang, Qiaowen Yang, Ting Li, Sifan Cui, Pengqiang Feng, Xuan Hou","doi":"10.1002/jctb.7738","DOIUrl":null,"url":null,"abstract":"<div>\n \n \n <section>\n \n <h3> BACKGROUND</h3>\n \n <p>Selective catalytic reduction (SCR) can effectively remove NO<sub><i>x</i></sub> from flue gas in a coal-fired power plant. As the core of SCR technology, the catalyst has a limited lifespan. The process of reducing acid leaching and roasting water leaching can efficiently extract vanadium and tungsten from spent SCR catalyst. For vanadium recovery in solution, the traditional vanadium precipitation process by NH<sub>4</sub>Cl has the disadvantages of consuming high precipitant dosage and producing large amounts of waste water.</p>\n </section>\n \n <section>\n \n <h3> RESULTS</h3>\n \n <p>This study focused on the vanadium precipitation process using a solution containing vanadium obtained from adopting some steps to handle the spent SCR catalyst. First, melamine was screened out as the vanadium precipitant. Then, the vanadium precipitation conditions were optimized as follows: solution pH value of 1.0, <i>n</i>(C<sub>3</sub>H<sub>6</sub>N<sub>6</sub>)/<i>n</i>(V) = 0.8, 110 °C and 30 min. Under the best precipitation conditions, the vanadium precipitation efficiency of melamine can reach 99.32%. Finally, the mechanisms of vanadium precipitation by NH<sub>4</sub>Cl and melamine were discussed, respectively.</p>\n </section>\n \n <section>\n \n <h3> CONCLUSION</h3>\n \n <p>The results showed that the vanadium precipitation process by NH<sub>4</sub>Cl followed the chemical reactions between NH<sub>4</sub><sup>+</sup> and vanadium ions, while the vanadium precipitation process by melamine followed the redox and complexation reactions which essentially belonged to the types of chemical adsorption. The vanadium precipitation product was roasted at 550 °C for 2 h to obtain the vanadium product. The main component of the vanadium product was V<sub>2</sub>O<sub>5</sub>, with a purity of 93.27 wt%. The total recovery efficiency of vanadium from spent SCR catalyst was 78.24%. © 2024 Society of Chemical Industry (SCI).</p>\n </section>\n </div>","PeriodicalId":15335,"journal":{"name":"Journal of chemical technology and biotechnology","volume":"99 12","pages":"2569-2581"},"PeriodicalIF":2.8000,"publicationDate":"2024-08-28","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"0","resultStr":"{\"title\":\"Rapid precipitation of vanadium from solution using melamine as precipitant with high efficiency\",\"authors\":\"Bo Wang, Qiaowen Yang, Ting Li, Sifan Cui, Pengqiang Feng, Xuan Hou\",\"doi\":\"10.1002/jctb.7738\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<div>\\n \\n \\n <section>\\n \\n <h3> BACKGROUND</h3>\\n \\n <p>Selective catalytic reduction (SCR) can effectively remove NO<sub><i>x</i></sub> from flue gas in a coal-fired power plant. As the core of SCR technology, the catalyst has a limited lifespan. The process of reducing acid leaching and roasting water leaching can efficiently extract vanadium and tungsten from spent SCR catalyst. For vanadium recovery in solution, the traditional vanadium precipitation process by NH<sub>4</sub>Cl has the disadvantages of consuming high precipitant dosage and producing large amounts of waste water.</p>\\n </section>\\n \\n <section>\\n \\n <h3> RESULTS</h3>\\n \\n <p>This study focused on the vanadium precipitation process using a solution containing vanadium obtained from adopting some steps to handle the spent SCR catalyst. First, melamine was screened out as the vanadium precipitant. Then, the vanadium precipitation conditions were optimized as follows: solution pH value of 1.0, <i>n</i>(C<sub>3</sub>H<sub>6</sub>N<sub>6</sub>)/<i>n</i>(V) = 0.8, 110 °C and 30 min. Under the best precipitation conditions, the vanadium precipitation efficiency of melamine can reach 99.32%. Finally, the mechanisms of vanadium precipitation by NH<sub>4</sub>Cl and melamine were discussed, respectively.</p>\\n </section>\\n \\n <section>\\n \\n <h3> CONCLUSION</h3>\\n \\n <p>The results showed that the vanadium precipitation process by NH<sub>4</sub>Cl followed the chemical reactions between NH<sub>4</sub><sup>+</sup> and vanadium ions, while the vanadium precipitation process by melamine followed the redox and complexation reactions which essentially belonged to the types of chemical adsorption. The vanadium precipitation product was roasted at 550 °C for 2 h to obtain the vanadium product. The main component of the vanadium product was V<sub>2</sub>O<sub>5</sub>, with a purity of 93.27 wt%. The total recovery efficiency of vanadium from spent SCR catalyst was 78.24%. © 2024 Society of Chemical Industry (SCI).</p>\\n </section>\\n </div>\",\"PeriodicalId\":15335,\"journal\":{\"name\":\"Journal of chemical technology and biotechnology\",\"volume\":\"99 12\",\"pages\":\"2569-2581\"},\"PeriodicalIF\":2.8000,\"publicationDate\":\"2024-08-28\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Journal of chemical technology and biotechnology\",\"FirstCategoryId\":\"5\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/jctb.7738\",\"RegionNum\":4,\"RegionCategory\":\"生物学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q3\",\"JCRName\":\"BIOTECHNOLOGY & APPLIED MICROBIOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Journal of chemical technology and biotechnology","FirstCategoryId":"5","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jctb.7738","RegionNum":4,"RegionCategory":"生物学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q3","JCRName":"BIOTECHNOLOGY & APPLIED MICROBIOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用