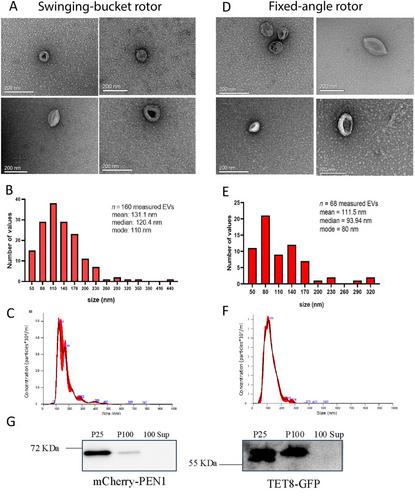
{"title":"从烟草叶的凋落洗液中分离出小细胞外囊泡 (sEVs)","authors":"Mahmoud K. Eldahshoury, Konstantina Katsarou, Joshua T. Farley, Kriton Kalantidis, Carine de Marcos Lousa","doi":"10.1002/cpz1.70026","DOIUrl":null,"url":null,"abstract":"<p>Extracellular vesicles (EVs) are small membranous vesicles secreted by cells into their surrounding extracellular environment. Similar to mammalian EVs, plant EVs have emerged as essential mediators of intercellular communication in plants that facilitate the transfer of biological material between cells. They also play essential roles in diverse physiological processes including stress responses, developmental regulation, and defense mechanisms against pathogens. In addition, plant EVs have demonstrated promising health benefits as well as potential therapeutic effects in mammalian health. Despite the plethora of potential applications using plant EVs, their isolation and characterization remains challenging. In contrast to mammalian EVs, which benefit from more standardized isolation protocols, methods for isolating plant EVs can vary depending on the starting material used, resulting in diverse levels of purity and composition. Additionally, the field suffers from the lack of plant EV markers. Nevertheless, three main EV subclasses have been described from leaf apoplasts: tetraspanin 8 positive (TET8), penetration-1-positive (PEN1), and EXPO vesicles derived from exocyst-positive organelles (EXPO). Here, we present an optimized protocol for the isolation and enrichment of small EVs (sEVs; <200 nm) from the apoplastic fluid from <i>Nicotiana benthamiana</i> leaves by ultracentrifugation. We analyze the preparation through transmitted electron microscopy (TEM), nanoparticle tracking analysis (NTA), and western blotting. We believe this method will establish a basic protocol for the isolation of EVs from <i>N. benthamiana</i> leaves, and we discuss technical considerations to be evaluated by each researcher working towards improving their plant sEV preparations. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol</b>: Isolation and enrichment of small extracellular vesicles (sEVs) from the apoplastic fluid of <i>Nicotiana benthamiana</i> leaves</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 11","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2024-11-05","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.70026","citationCount":"0","resultStr":"{\"title\":\"Isolation of Small Extracellular Vesicles (sEVs) from the Apoplastic Wash Fluid of Nicotiana benthamiana Leaves\",\"authors\":\"Mahmoud K. Eldahshoury, Konstantina Katsarou, Joshua T. Farley, Kriton Kalantidis, Carine de Marcos Lousa\",\"doi\":\"10.1002/cpz1.70026\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Extracellular vesicles (EVs) are small membranous vesicles secreted by cells into their surrounding extracellular environment. Similar to mammalian EVs, plant EVs have emerged as essential mediators of intercellular communication in plants that facilitate the transfer of biological material between cells. They also play essential roles in diverse physiological processes including stress responses, developmental regulation, and defense mechanisms against pathogens. In addition, plant EVs have demonstrated promising health benefits as well as potential therapeutic effects in mammalian health. Despite the plethora of potential applications using plant EVs, their isolation and characterization remains challenging. In contrast to mammalian EVs, which benefit from more standardized isolation protocols, methods for isolating plant EVs can vary depending on the starting material used, resulting in diverse levels of purity and composition. Additionally, the field suffers from the lack of plant EV markers. Nevertheless, three main EV subclasses have been described from leaf apoplasts: tetraspanin 8 positive (TET8), penetration-1-positive (PEN1), and EXPO vesicles derived from exocyst-positive organelles (EXPO). Here, we present an optimized protocol for the isolation and enrichment of small EVs (sEVs; <200 nm) from the apoplastic fluid from <i>Nicotiana benthamiana</i> leaves by ultracentrifugation. We analyze the preparation through transmitted electron microscopy (TEM), nanoparticle tracking analysis (NTA), and western blotting. We believe this method will establish a basic protocol for the isolation of EVs from <i>N. benthamiana</i> leaves, and we discuss technical considerations to be evaluated by each researcher working towards improving their plant sEV preparations. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol</b>: Isolation and enrichment of small extracellular vesicles (sEVs) from the apoplastic fluid of <i>Nicotiana benthamiana</i> leaves</p>\",\"PeriodicalId\":93970,\"journal\":{\"name\":\"Current protocols\",\"volume\":\"4 11\",\"pages\":\"\"},\"PeriodicalIF\":0.0000,\"publicationDate\":\"2024-11-05\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.70026\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Current protocols\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.70026\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"\",\"JCRName\":\"\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.70026","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0