{"title":"利用人原代包皮成纤维细胞研究细胞损伤和线粒体功能障碍","authors":"Cristina A. Nadalutti, Samuel H. Wilson","doi":"10.1002/cptx.99","DOIUrl":null,"url":null,"abstract":"<p>Several cell lines of different origin are routinely used in research and drug development as important models to study human health and disease. Studying cells in culture represents an easy and convenient tool to approach complex biological questions, but the disadvantage is that they may not necessarily reflect what is effectively occurring in vivo. Human primary cells can help address this limitation, as they are isolated directly from human biological samples and can preserve the morphological and functional features of their tissue of origin. In addition, these can offer more relevant data and better solutions to investigators because they are not genetically manipulated. Human foreskin tissue discarded after surgery, for instance, represents a precious source for isolating such cells, including human foreskin fibroblasts (FSK), which are used in several areas of research and medicine. The overall health of cells is determined by the mitochondria. Alterations of cellular metabolism and cell death pathways depend, in part, on the number, size, distribution, and structure of mitochondria, and these can change under different cellular and pathological conditions. This highlights the need to develop accurate approaches to study mitochondria and evaluate their function. Here, we describe three easy, step-by-step protocols to study cellular viability and mitochondrial functionality in FSK. We describe how to use circumcision tissue obtained from the clinic to isolate FSK cells by mechanical and enzymatic disaggregation, how to use a cationic dye, crystal violet, which is retained by proliferating cells, to determine cell viability, and how to prepare samples to assess the metabolic status of cells by evaluating different mitochondrial parameters with transmission electron microscopy. We have successfully used the approaches outlined here to recapitulate physiological conditions in these cells in order to study the effects of increased intracellular levels of formaldehyde. © 2020 U.S. Government.</p><p><b>Basic Protocol 1</b>: Isolation and maintenance of human primary foreskin fibroblasts (FSK)</p><p><b>Basic Protocol 2</b>: Determination of cell viability by crystal violet staining</p><p><b>Basic Protocol 3</b>: Transmission electron microscopy to study cellular damage and mitochondrial dysfunction</p>","PeriodicalId":72743,"journal":{"name":"Current protocols in toxicology","volume":"86 1","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2020-11-17","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cptx.99","citationCount":"3","resultStr":"{\"title\":\"Using Human Primary Foreskin Fibroblasts to Study Cellular Damage and Mitochondrial Dysfunction\",\"authors\":\"Cristina A. Nadalutti, Samuel H. Wilson\",\"doi\":\"10.1002/cptx.99\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Several cell lines of different origin are routinely used in research and drug development as important models to study human health and disease. Studying cells in culture represents an easy and convenient tool to approach complex biological questions, but the disadvantage is that they may not necessarily reflect what is effectively occurring in vivo. Human primary cells can help address this limitation, as they are isolated directly from human biological samples and can preserve the morphological and functional features of their tissue of origin. In addition, these can offer more relevant data and better solutions to investigators because they are not genetically manipulated. Human foreskin tissue discarded after surgery, for instance, represents a precious source for isolating such cells, including human foreskin fibroblasts (FSK), which are used in several areas of research and medicine. The overall health of cells is determined by the mitochondria. Alterations of cellular metabolism and cell death pathways depend, in part, on the number, size, distribution, and structure of mitochondria, and these can change under different cellular and pathological conditions. This highlights the need to develop accurate approaches to study mitochondria and evaluate their function. Here, we describe three easy, step-by-step protocols to study cellular viability and mitochondrial functionality in FSK. We describe how to use circumcision tissue obtained from the clinic to isolate FSK cells by mechanical and enzymatic disaggregation, how to use a cationic dye, crystal violet, which is retained by proliferating cells, to determine cell viability, and how to prepare samples to assess the metabolic status of cells by evaluating different mitochondrial parameters with transmission electron microscopy. We have successfully used the approaches outlined here to recapitulate physiological conditions in these cells in order to study the effects of increased intracellular levels of formaldehyde. © 2020 U.S. Government.</p><p><b>Basic Protocol 1</b>: Isolation and maintenance of human primary foreskin fibroblasts (FSK)</p><p><b>Basic Protocol 2</b>: Determination of cell viability by crystal violet staining</p><p><b>Basic Protocol 3</b>: Transmission electron microscopy to study cellular damage and mitochondrial dysfunction</p>\",\"PeriodicalId\":72743,\"journal\":{\"name\":\"Current protocols in toxicology\",\"volume\":\"86 1\",\"pages\":\"\"},\"PeriodicalIF\":0.0000,\"publicationDate\":\"2020-11-17\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://sci-hub-pdf.com/10.1002/cptx.99\",\"citationCount\":\"3\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Current protocols in toxicology\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/cptx.99\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"\",\"JCRName\":\"\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols in toxicology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cptx.99","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 3
Using Human Primary Foreskin Fibroblasts to Study Cellular Damage and Mitochondrial Dysfunction
Several cell lines of different origin are routinely used in research and drug development as important models to study human health and disease. Studying cells in culture represents an easy and convenient tool to approach complex biological questions, but the disadvantage is that they may not necessarily reflect what is effectively occurring in vivo. Human primary cells can help address this limitation, as they are isolated directly from human biological samples and can preserve the morphological and functional features of their tissue of origin. In addition, these can offer more relevant data and better solutions to investigators because they are not genetically manipulated. Human foreskin tissue discarded after surgery, for instance, represents a precious source for isolating such cells, including human foreskin fibroblasts (FSK), which are used in several areas of research and medicine. The overall health of cells is determined by the mitochondria. Alterations of cellular metabolism and cell death pathways depend, in part, on the number, size, distribution, and structure of mitochondria, and these can change under different cellular and pathological conditions. This highlights the need to develop accurate approaches to study mitochondria and evaluate their function. Here, we describe three easy, step-by-step protocols to study cellular viability and mitochondrial functionality in FSK. We describe how to use circumcision tissue obtained from the clinic to isolate FSK cells by mechanical and enzymatic disaggregation, how to use a cationic dye, crystal violet, which is retained by proliferating cells, to determine cell viability, and how to prepare samples to assess the metabolic status of cells by evaluating different mitochondrial parameters with transmission electron microscopy. We have successfully used the approaches outlined here to recapitulate physiological conditions in these cells in order to study the effects of increased intracellular levels of formaldehyde. © 2020 U.S. Government.
Basic Protocol 1: Isolation and maintenance of human primary foreskin fibroblasts (FSK)
Basic Protocol 2: Determination of cell viability by crystal violet staining
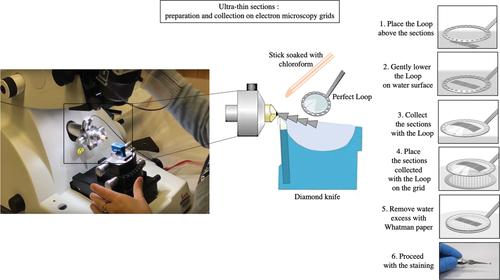
Basic Protocol 3: Transmission electron microscopy to study cellular damage and mitochondrial dysfunction