Age-related sarcopenia, characterized by reduced skeletal muscle mass and function, significantly affects the health of the elderly individuals. Oxidative stress plays a crucial role in the development of sarcopenia. Tripartite motif containing 16 (TRIM16) is implicated in orchestrating antioxidant responses to mitigate oxidative stress, yet its regulatory role in skeletal muscle remains unclear. This study aims to elucidate the impact of TRIM16 on enhancing antioxidant response through SIRT-1, consequently mitigating age-related oxidative stress, and ameliorating muscle atrophy.
Aged mouse models were established utilizing male mice at 18 months with D-galactose (D-gal, 200 mg/kg) intervention and at 24 months with natural aging, while 3-month-old young mice served as controls. Muscle cell senescence was induced in C2C12 myoblasts using 30 g/L D-gal. TRIM16 was overexpressed in the skeletal muscle of aged mice and silenced/overexpressed in C2C12 myoblasts. The effects of TRIM16 on skeletal muscle mass, grip strength, morphological changes, myotube formation, myogenic differentiation, and muscle atrophy indicators were evaluated. Reactive oxygen species (ROS) levels and oxidative stress-related parameters were measured. The SIRT-1 inhibitor EX-527 was employed to elucidate the protective role of TRIM16 mediated through SIRT-1.
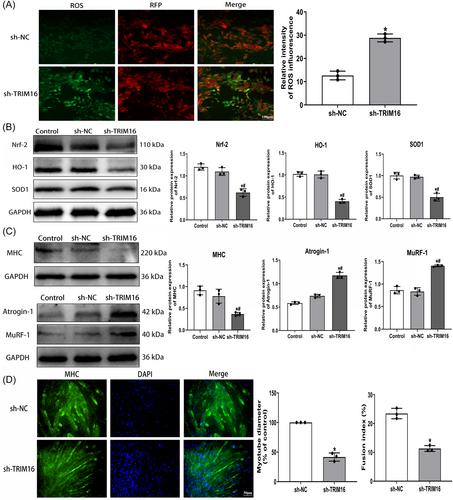
Aged mice displayed significant reductions in lean mass (−11.58%; −14.47% vs. young, P < 0.05), hindlimb lean mass (−17.38%; −15.95% vs. young, P < 0.05), and grip strength (−22.29%; −31.45% vs. young, P < 0.01). Skeletal muscle fibre cross-sectional area (CSA) decreased (−29.30%; −24.12% vs. young, P < 0.05). TRIM16 expression significantly decreased in aging skeletal muscle (−56.82%; −66.27% vs. young, P < 0.001) and senescent muscle cells (−46.53% vs. control, P < 0.001). ROS levels increased (+69.83% vs. control, P < 0.001), and myotube formation decreased in senescent muscle cells (−56.68% vs. control, P < 0.001). Expression of myogenic differentiation and antioxidant indicators decreased, while muscle atrophy markers increased in vivo and in vitro (all P < 0.05). Silencing TRIM16 in myoblasts induced oxidative stress and myotube atrophy, while TRIM16 overexpression partially mitigated aging effects on skeletal muscle. TRIM16 activation enhanced SIRT-1 expression (+75.38% vs. control, P < 0.001). SIRT-1 inhibitor EX-527 (100 μM) suppressed TRIM16's antioxidant response and mitigating muscle atrophy, offsetting the protective effect of TRIM16 on senescent muscle cells.
This study elucidates TRIM16's role in mitigating oxidative stress and ameliorating muscle atrophy through the activation of SIRT-1-dependent antioxidant effects. TRIM16 emerges as a potential therapeutic target for age-related sarcopenia.