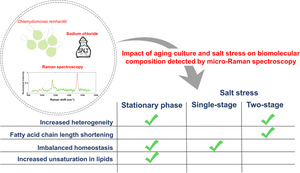
Salt stress on green microalgae increases lipid production at the cost of cellular homeostasis. Rapid optimization of growth conditions for high lipid productivity and biomass yield is crucial for translation to industrial-scale biodiesel production. To achieve this, the present study has developed a spectroscopic non-invasive analysis of lipid molecules produced by Chlamydomonas reinhardtii in two-stage salt stress, wherein 100 mM NaCl was added at two different time points: day 2 (D2 100) and day 4 (D4 100) of growth. Two-stage stress resulted in cell morphology like the photoautotrophic control grown in normal conditions, with negligible palmelloid formation in contrast to single-stage. Raman spectra acquired from ~ 30 individual cells in each culture revealed heterogeneities in lipid composition. Discrete wavelet transform decomposition of the Raman signal was used to enhance the signal-to-noise ratio and accuracy of Raman peak center estimation. An overall increase in heterogeneity indices for fatty acid degree of unsaturation was observed under two-stage salt stress: fourfold for D2 100 and ninefold for D4 100, especially at the stationary growth phase. The ratio of the CH2/CH3 scissoring mode (1440 cm−1) and the C = O stretching mode (1750 cm−1) reveals the shortening of fatty acid chain length in D4 100. Although Raman bands of lipids formed in all growth conditions are on average like Triolein (18:1), analyses of the degree of unsaturation (1656/1440 cm−1) clarify the increased content of bi and tri-unsaturation only in D4 100. This non-invasive lipid profiling reveals that D4 100 is likely a non-ideal condition to obtain high-quality biodiesel-producing lipids. A comparative analysis of single-cell fluorescence microscopy of lipid droplets and Raman intensity of lipids shows the sensitivity of Raman intensity in deciphering the relative response of the cells to salt stress.