下载PDF
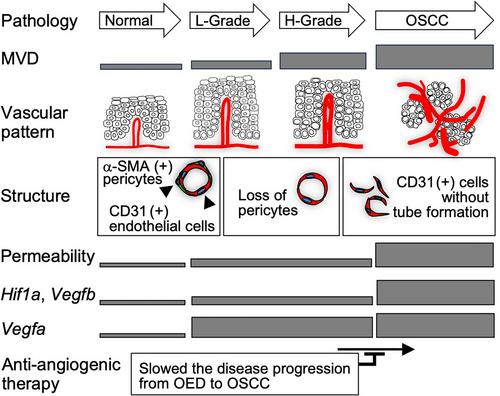
{"title":"Anti-VEGFR2 neutralising antibody slows the progression of multistep oral carcinogenesis","authors":"Yoichiro Shirogane, Yu Usami, Masashi Okumura, Katsutoshi Hirose, Kohei Naniwa, Kazunori Ikebe, Satoru Toyosawa","doi":"10.1002/path.6357","DOIUrl":null,"url":null,"abstract":"<p>Angiogenesis plays an important role in cancer growth and metastasis, and it is considered a therapeutic target to control tumour growth following anti-angiogenic therapy. However, it is still unclear when tissues initiate angiogenesis during malignant transformation from premalignant condition and whether this premalignant condition could be a therapeutic target of anti-angiogenic therapy. In this study, we aimed to analyse the onset of angiogenesis by evaluating morphological and functional alterations of microvessels during oral multistep carcinogenesis using a 4-nitroquinoline 1-oxide (4NQO)-induced oral carcinogenesis mouse model. In the study, we initially confirmed that with the use of 4NQO, oral lesions develop in a stepwise manner from normal mucosa through oral epithelial dysplasia (OED) to oral squamous cell carcinoma (OSCC). Evaluation of CD31-immunostained specimens revealed that microvessel density (MVD) increases in a stepwise manner from OEDs. Histological and functional analyses revealed the structural abnormalities and leakage of blood vessels had already taken place in OED. Then we evaluated the expression profiles of <i>Hif1a</i> and <i>Vegfa</i> along with hypoxic status and found that OED exhibited increased <i>Vegfa</i> expression under hypoxic conditions. Finally, we tested the possibility of OEDs as a target of anti-angiogenic therapy and found that anti-VEGFR2 neutralising antibody in OED slowed the disease progression from OED to OSCC. These data indicate that an angiogenic switch occurs at the premalignant stage and morphological, and functional alterations of microvessels already exist in OED. These findings also elucidate the tumour microenvironment, which gradually develops along with carcinogenic processes, and highlight usefulness of the 4NQO-induced carcinogenesis model in the study of epithelial and stromal components, which will support epithelial carcinogenesis. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 4","pages":"423-433"},"PeriodicalIF":5.6000,"publicationDate":"2024-10-27","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6357","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6357","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
Angiogenesis plays an important role in cancer growth and metastasis, and it is considered a therapeutic target to control tumour growth following anti-angiogenic therapy. However, it is still unclear when tissues initiate angiogenesis during malignant transformation from premalignant condition and whether this premalignant condition could be a therapeutic target of anti-angiogenic therapy. In this study, we aimed to analyse the onset of angiogenesis by evaluating morphological and functional alterations of microvessels during oral multistep carcinogenesis using a 4-nitroquinoline 1-oxide (4NQO)-induced oral carcinogenesis mouse model. In the study, we initially confirmed that with the use of 4NQO, oral lesions develop in a stepwise manner from normal mucosa through oral epithelial dysplasia (OED) to oral squamous cell carcinoma (OSCC). Evaluation of CD31-immunostained specimens revealed that microvessel density (MVD) increases in a stepwise manner from OEDs. Histological and functional analyses revealed the structural abnormalities and leakage of blood vessels had already taken place in OED. Then we evaluated the expression profiles of Hif1a and Vegfa along with hypoxic status and found that OED exhibited increased Vegfa expression under hypoxic conditions. Finally, we tested the possibility of OEDs as a target of anti-angiogenic therapy and found that anti-VEGFR2 neutralising antibody in OED slowed the disease progression from OED to OSCC. These data indicate that an angiogenic switch occurs at the premalignant stage and morphological, and functional alterations of microvessels already exist in OED. These findings also elucidate the tumour microenvironment, which gradually develops along with carcinogenic processes, and highlight usefulness of the 4NQO-induced carcinogenesis model in the study of epithelial and stromal components, which will support epithelial carcinogenesis. © 2024 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
抗血管内皮生长因子受体 2(VEGFR2)中和抗体可延缓多步口腔癌变的进程。
血管生成在癌症生长和转移中发挥着重要作用,被认为是抗血管生成疗法后控制肿瘤生长的治疗靶点。然而,组织在从恶性前状态向恶性转化的过程中何时开始血管生成,以及这种恶性前状态能否成为抗血管生成疗法的治疗靶点,目前仍不清楚。在本研究中,我们利用 4-硝基喹啉-1-氧化物(4NQO)诱导的口腔癌小鼠模型,通过评估口腔多步癌变过程中微血管的形态和功能变化,分析血管生成的起始时间。在这项研究中,我们初步证实了使用 4NQO 后,口腔病变会以循序渐进的方式从正常粘膜发展到口腔上皮发育不良(OED)再到口腔鳞状细胞癌(OSCC)。对 CD31 免疫染色标本的评估显示,从 OED 开始,微血管密度(MVD)逐步增加。组织学和功能学分析表明,OED已出现结构异常和血管渗漏。然后,我们评估了缺氧状态下Hif1a和Vegfa的表达谱,发现OED在缺氧条件下Vegfa的表达增加。最后,我们测试了将 OED 作为抗血管生成治疗靶点的可能性,发现在 OED 中使用抗血管内皮生长因子受体 2(VEGFR2)中和抗体可减缓从 OED 到 OSCC 的疾病进展。这些数据表明,血管生成的转换发生在恶性肿瘤的前期阶段,OED中已经存在微血管的形态和功能改变。这些研究结果还阐明了随着致癌过程逐渐发展的肿瘤微环境,并强调了4NQO诱导致癌模型在研究上皮和基质成分方面的有用性,这将支持上皮癌变。© 2024 作者。病理学杂志》由 John Wiley & Sons Ltd 代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。