Endogenous retroelements (EREs) stimulate type 1 interferon (IFN-I) production but have not been explored as potential interferonogenic triggers in rheumatoid arthritis (RA). We investigated ERE expression in early RA (eRA), a period in which IFN-I levels are increased.
ERE expression (long terminal repeat [LTR] 5, long interspersed nuclear element 1 [LINE-1], and short interspersed nuclear element [SINE]) in disease-modifying treatment-naïve eRA whole-blood and bulk synovial tissue samples was examined by reverse transcription–polymerase chain reaction and NanoString alongside IFN-α activity. Circulating lymphocyte subsets, including B cell subsets, from patients with eRA and early psoriatic arthritis (ePsA) were flow cytometrically sorted and similarly examined. Existing established RA and osteoarthritis (OA) synovial single-cell sequencing data were reinterrogated to identify repeat elements, and associations were explored.
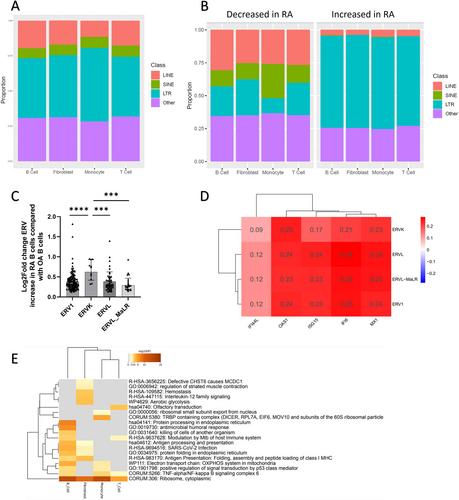
There was significant coexpression of all ERE classes and IFNA in eRA synovial tissue samples (n = 22, P < 0.0001) and significant positive associations between whole-blood LINE-1 expression (n = 56) and circulating IFN-α protein (P = 0.018) and anti–cyclic citrullinated peptide (anti-CCP) titers (P < 0.0001). ERE expression was highest in circulating eRA B cells, particularly naïve B cells compared with ePsA, with possible ERE regulation by SAM and HD Domain Containing Deoxynucleoside Triphosphate Triphosphohydrolase 1 transcription (SAMDH1) implicated and associations with IFNA again observed. Finally, in established RA synovium, LTRs, particularly human endogenous retroviral sequence K (HERVK), were most increased in RA compared with OA, in which, for all synovial subsets (monocytes, B cells, T cells, and fibroblasts), ERE expression associated with increased IFN-I signaling (P < 0.001).
Peripheral blood and synovial ERE expression is examined for the first time in eRA, highlighting both a potential causal relationship between ERE and IFN-I production and an intriguing association with anti-CCP autoantibodies. This suggests EREs may contribute to RA pathophysiology with implications for future novel therapeutic strategies.