Development of a Rapid Focus Reduction Neutralization Test Assay for Measuring SARS-CoV-2 Neutralizing Antibodies
Abigail Vanderheiden, Venkata Viswanadh Edara, Katharine Floyd, Robert C. Kauffman, Grace Mantus, Evan Anderson, Nadine Rouphael, Sri Edupuganti, Pei-Yong Shi, Vineet D. Menachery, Jens Wrammert, Mehul S. Suthar
{"title":"Development of a Rapid Focus Reduction Neutralization Test Assay for Measuring SARS-CoV-2 Neutralizing Antibodies","authors":"Abigail Vanderheiden, Venkata Viswanadh Edara, Katharine Floyd, Robert C. Kauffman, Grace Mantus, Evan Anderson, Nadine Rouphael, Sri Edupuganti, Pei-Yong Shi, Vineet D. Menachery, Jens Wrammert, Mehul S. Suthar","doi":"10.1002/cpim.116","DOIUrl":null,"url":null,"abstract":"SARS‐CoV‐2 is a recently emerged human coronavirus that has escalated to a pandemic. There are currently no approved vaccines for SARS‐CoV‐2, which causes severe respiratory illness or death. Defining the antibody response to SARS‐CoV‐2 will be essential for understanding disease progression, long‐term immunity, and vaccine efficacy. Here we describe two methods for evaluating the neutralization capacity of SARS‐CoV‐2 antibodies. The basic protocol is a focus reduction neutralization test (FRNT), which involves immunostaining infected cells with a chromogen deposit readout. The alternate protocol is a modification of the FRNT that uses an infectious clone−derived SARS‐CoV‐2 virus expressing a fluorescent reporter. These protocols are adapted for use in a high‐throughput setting, and are compatible with large‐scale vaccine studies or clinical testing. © 2020 Wiley Periodicals LLC","PeriodicalId":10733,"journal":{"name":"Current Protocols in Immunology","volume":"131 1","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2020-11-20","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cpim.116","citationCount":"101","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current Protocols in Immunology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpim.116","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"Immunology and Microbiology","Score":null,"Total":0}
引用次数: 101
Abstract
SARS‐CoV‐2 is a recently emerged human coronavirus that has escalated to a pandemic. There are currently no approved vaccines for SARS‐CoV‐2, which causes severe respiratory illness or death. Defining the antibody response to SARS‐CoV‐2 will be essential for understanding disease progression, long‐term immunity, and vaccine efficacy. Here we describe two methods for evaluating the neutralization capacity of SARS‐CoV‐2 antibodies. The basic protocol is a focus reduction neutralization test (FRNT), which involves immunostaining infected cells with a chromogen deposit readout. The alternate protocol is a modification of the FRNT that uses an infectious clone−derived SARS‐CoV‐2 virus expressing a fluorescent reporter. These protocols are adapted for use in a high‐throughput setting, and are compatible with large‐scale vaccine studies or clinical testing. © 2020 Wiley Periodicals LLC
用于检测SARS-CoV-2中和抗体的快速减焦中和试验方法的建立
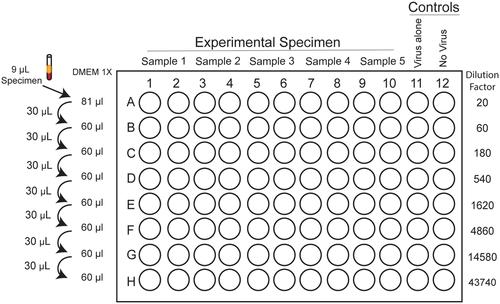
SARS-CoV-2是最近出现的一种人类冠状病毒,已升级为大流行。目前还没有批准的SARS-CoV-2疫苗,这种疫苗会导致严重的呼吸道疾病或死亡。确定对SARS-CoV-2的抗体反应对于了解疾病进展、长期免疫和疫苗功效至关重要。本文介绍了评估SARS-CoV-2抗体中和能力的两种方法。基本方案是焦点减少中和试验(FRNT),包括用色素沉积读数对感染细胞进行免疫染色。替代方案是对FRNT的修改,使用表达荧光报告基因的传染性克隆衍生的SARS-CoV-2病毒。这些方案适用于高通量环境,并与大规模疫苗研究或临床试验兼容。©2020 Wiley期刊LLCBasic协议:减焦中和测试备用协议:基于mneongreen的减焦中和测试(FRNT-mNG)
本文章由计算机程序翻译,如有差异,请以英文原文为准。



