Gut microbiota plays a key role in the development of sarcopenia via the ‘gut-muscle’ axis, and probiotics-based therapy might be a strategy for sarcopenia. Fecal microbiota transplantation from young donors (yFMT) has attracted much attention because of its probiotic function. However, whether or not yFMT is effective for sarcopenia in old recipients is largely unknown. Thus, we aimed to investigate the effect and mechanism of yFMT on age-related sarcopenia.
The fecal microbiota of either young (12 weeks) or old (88 weeks) donor rats was transplanted into aged recipient rats for 8 weeks. Then, muscle mass, muscle strength, muscle function, muscle atrophy, and muscle regeneration capacity were measured. Analysis of fecal 16 s rRNA, serum non-targeted metabolomic, gut barrier integrity, and muscle transcriptome was conducted to elucidate the interaction between gut microbiota and skeletal muscles.
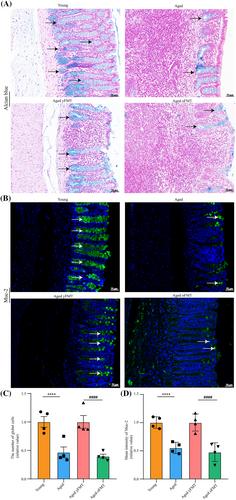
As evaluated by magnetic resonance imaging examination, grip strength test (P < 0.01), rotarod test (P < 0.05), and exhaustive running test (P < 0.05), we found that yFMT mitigated muscle mass loss, muscle strength weakness, and muscle function impairment in aged rats. yFMT also countered age-related atrophy and poor regeneration capacity in fast- and slow-switch muscles, which were manifested by the decrease in slow-switch myofibres (both P < 0.01) and muscle interstitial fibrosis (both P < 0.05) and the increase in the cross-section area of myofibres (both P < 0.001), fast-switch myofibres (both P < 0.01), and muscle satellite cells (both P < 0.001). In addition, yFMT ameliorated age-related dysbiosis of gut microbiota and metabolites by promoting the production of beneficial bacteria and metabolites—Akkermansia, Lactococcus, Lactobacillus, γ-glutamyltyrosine, 3R-hydroxy-butanoic acid, and methoxyacetic acid and inhibiting the production of deleterious bacteria and metabolites—Family_XIII_AD3011_group, Collinsella, indoxyl sulfate, indole-3-carboxilic acid-O-sulphate, and trimethylamine N-oxide. Also, yFMT prevented age-related destruction of gut barrier integrity by increasing the density of goblet cells (P < 0.0001) and the expression levels of mucin-2 (P < 0.0001) and tight junctional proteins (all P < 0.05). Meanwhile, yFMT attenuated age-related impairment of mitochondrial biogenesis and function in fast- and slow-switch muscles. Correlation analysis revealed that yFMT-induced alterations of gut microbiota and metabolites might be closely related to mitochondria-related genes and sarcopenia-related phenotypes.
yFMT could reshape the dysbiosis of gut microbiota and metabolites, maintain gut barrier integrity, and improve muscle mitochondrial dysfunction, eventually alleviating sarcopenia in aged rats. yFMT might be a new therapeutic strategy for age-related sarcopenia.